Rhodococcus ZJPH1003 and application thereof in preparing S-(+)-2,2-dimethylcyclopropane carboxylic acid
A technology of ethyl dimethylcyclopropanecarboxylate and dimethylcyclopropane is applied to Rhodococcus ZJPH1003 and its application field in the preparation of S-(+)-2,2-dimethylcyclopropanecarboxylic acid, which can Solve the problems of limited industrial application and high price of commercial lipase, and achieve the effects of shortened substrate conversion reaction time, easy cultivation and low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: the detection method of product
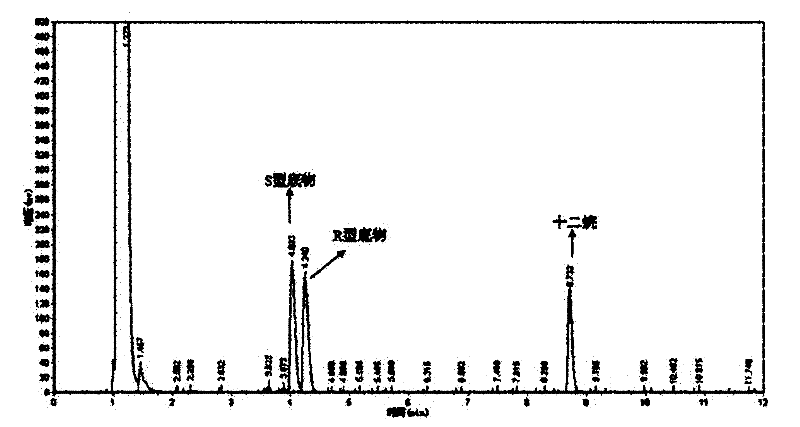
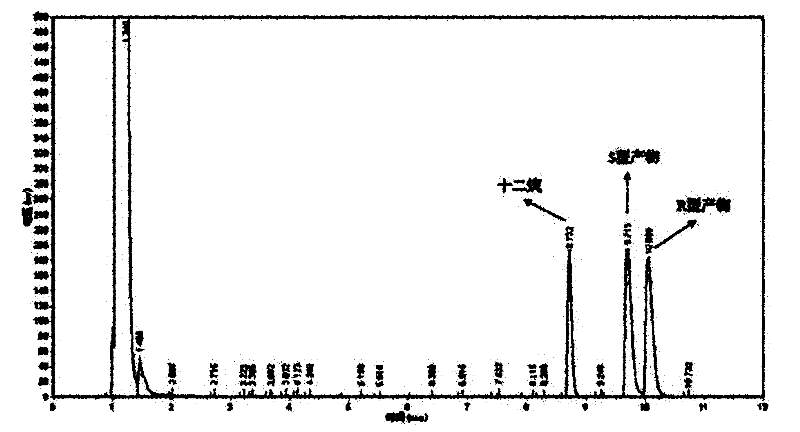
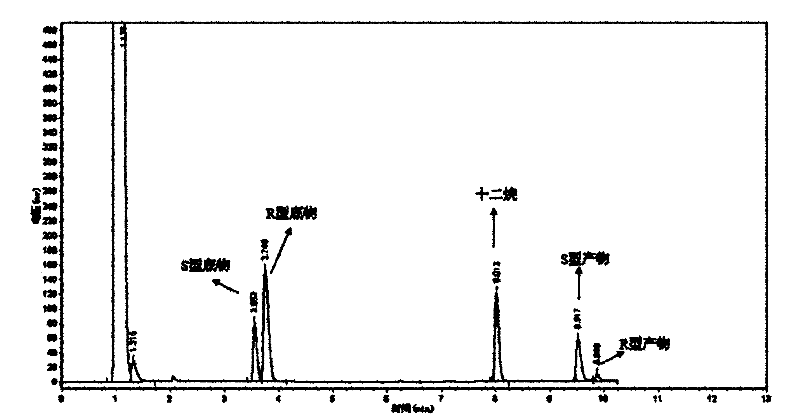
[0049] The optical purity and yield of the target product were determined by GC: the concentration of the product and residual substrate in the reaction extract was analyzed by gas chromatography, and quantified by the internal standard method. The internal standard is dodecane. Take 1 mL of the extract and add 1 μL of dodecane for analysis. Gas chromatography conditions: Japan Shimadzu GC-2014 gas chromatograph; American Varian CP-Chirasil-Dex chiral capillary gas chromatography column (25m×0.25mm×0.25μm). The carrier gas is high-purity nitrogen, the flow rate is 2mL / min; the injection volume is 1 μL, and the split ratio is 15:1; the temperature of the detector and the injection port are both 250°C; the temperature of the chromatographic column is 80-180°C; the heating rate: 8°C / min; the detector is FID.
[0050] The concentrations of substrate and product in the reaction solution were calculated using relative correc...
Embodiment 2
[0056] Embodiment 2: the acquisition of wet fungus cells
[0057] Seed medium formula: glucose 10g / L, peptone 5g / L, yeast extract 3g / L, (NH 4 ) 2 SO 4 4g / L, K 2 HPO 4 2g / L, KH 2 PO 4 1g / L, NaCl 0.5g / L, MgSO 4 ·7H 2 O0.5g / L, solvent is water, pH 7.0.
[0058] Fermentation medium formula: glycerol 15g / L, peptone 25g / L, K 2 HPO 4 2g / L, KH 2 PO 4 1g / L, NaCl 1.5g / L, MgSO 4 ·7H 2 O 0.5g / L, solvent is water, pH 7.0.
[0059] Pick a ring of bacteria (CCTCC NO: M 2010371) from the mature slant and insert it into a 250mL shake flask with 100mL seed medium, cultivate it at 30°C and 200rpm for 24 hours to obtain a seed solution, and then inoculate it with a volume ratio of 10%. Transfer the seed solution to a 250mL shake flask containing 100mL of fermentation medium, and culture at 30°C and 200rpm for 48 hours. After the cultivation, the fermented liquid was centrifuged and washed once with normal saline, and the wet bacterial cells were collected for future use.
Embodiment 3
[0061] The wet thalline obtained in Example 2 is suspended in 10 mL of disodium hydrogen phosphate-potassium dihydrogen phosphate buffer (pH 6.98), and the wet thalline is 400 g / L by wet weight; add 10 mmol / L of 2,2 -Ethyl dimethylcyclopropanecarboxylate was used as a substrate, and placed in a shaker at 20° C. at 200 rpm for 24 hours. Using the detection method of Example 1, the concentration of the product S-(+)-2,2-dimethylcyclopropanecarboxylic acid was 4.65mmol / L, the optical purity e.e. value was 80.1%, and the yield was 46.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com