Levofloxacin hydrochloride micropill capsule and preparation method thereof
A technology of levofloxacin hydrochloride and pellet capsules is applied in the field of medicine to achieve the effects of improving drug stability, reducing irritation and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
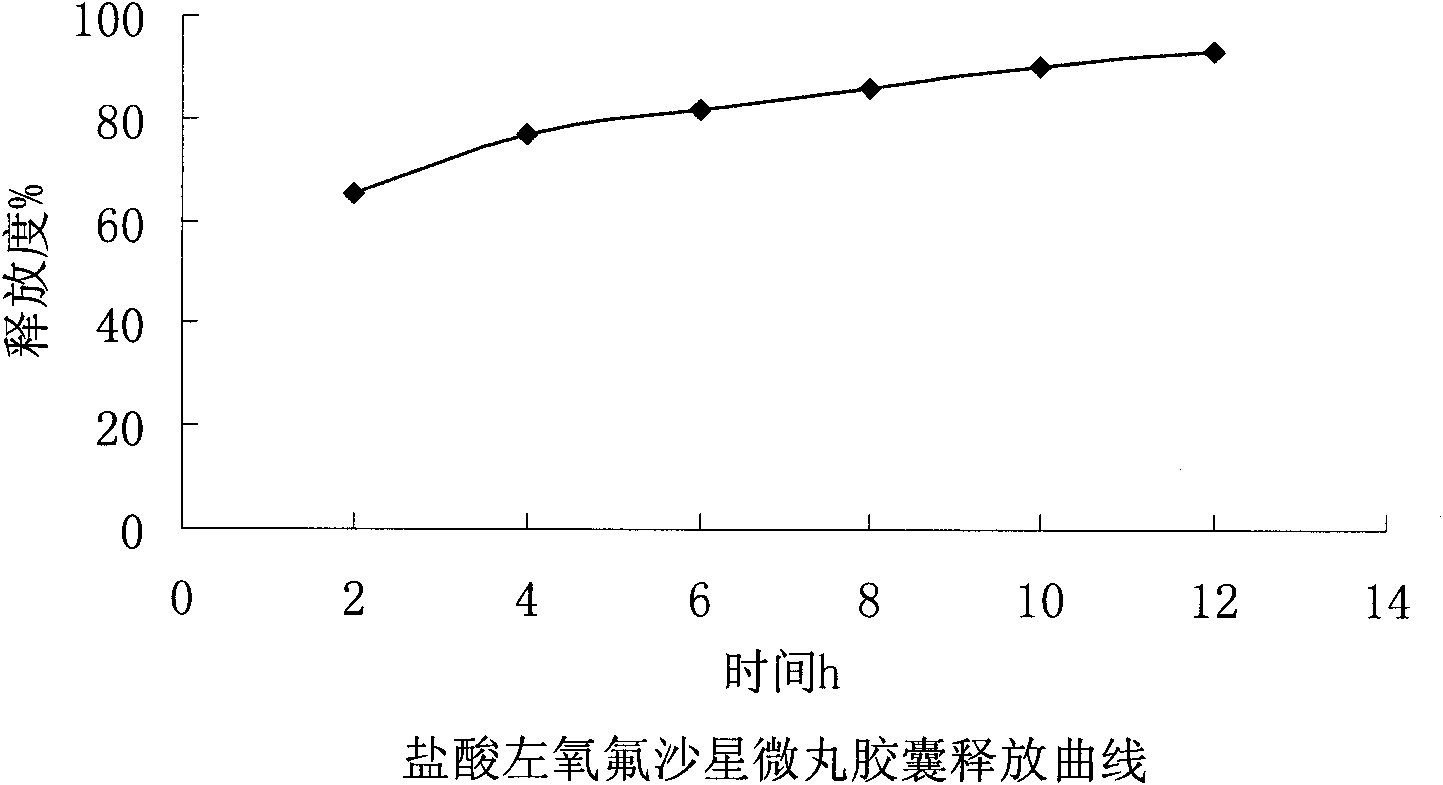
Embodiment 1
[0032] Embodiment 1: the present embodiment adopts following prescription:
[0033] Levofloxacin hydrochloride (calculated as ofloxacin) 200g
[0034] Sucrose core 153.4g
[0035] povidone k30 18.4g
[0036] Ethylcellulose 11.2g
[0037] Triethyl citrate 2.2g
[0038] Polyethylene glycol 6000 3.3g
[0039] Appropriate amount of talcum powder
[0040] Makes 1000 capsules
[0041] Preparation Process:
[0042] (1) Preparation of pill core:
[0043] First, 9.2g of povidone k30 is dissolved in the ethanol solution, 100g of levofloxacin hydrochloride is added in the povidone ethanol solution, and constantly stirred to make it into a uniform suspension; 153.4g of sucrose ball cores are placed in a fluidized bed In the granulator, the above-mentioned homogeneous suspension is sprayed on the surface of the blank sucrose pellet core to prepare pellets, and after all the suspension is added, the pellets are dried and screened;
[0044] (2) Sustained-release coating layer:
[0...
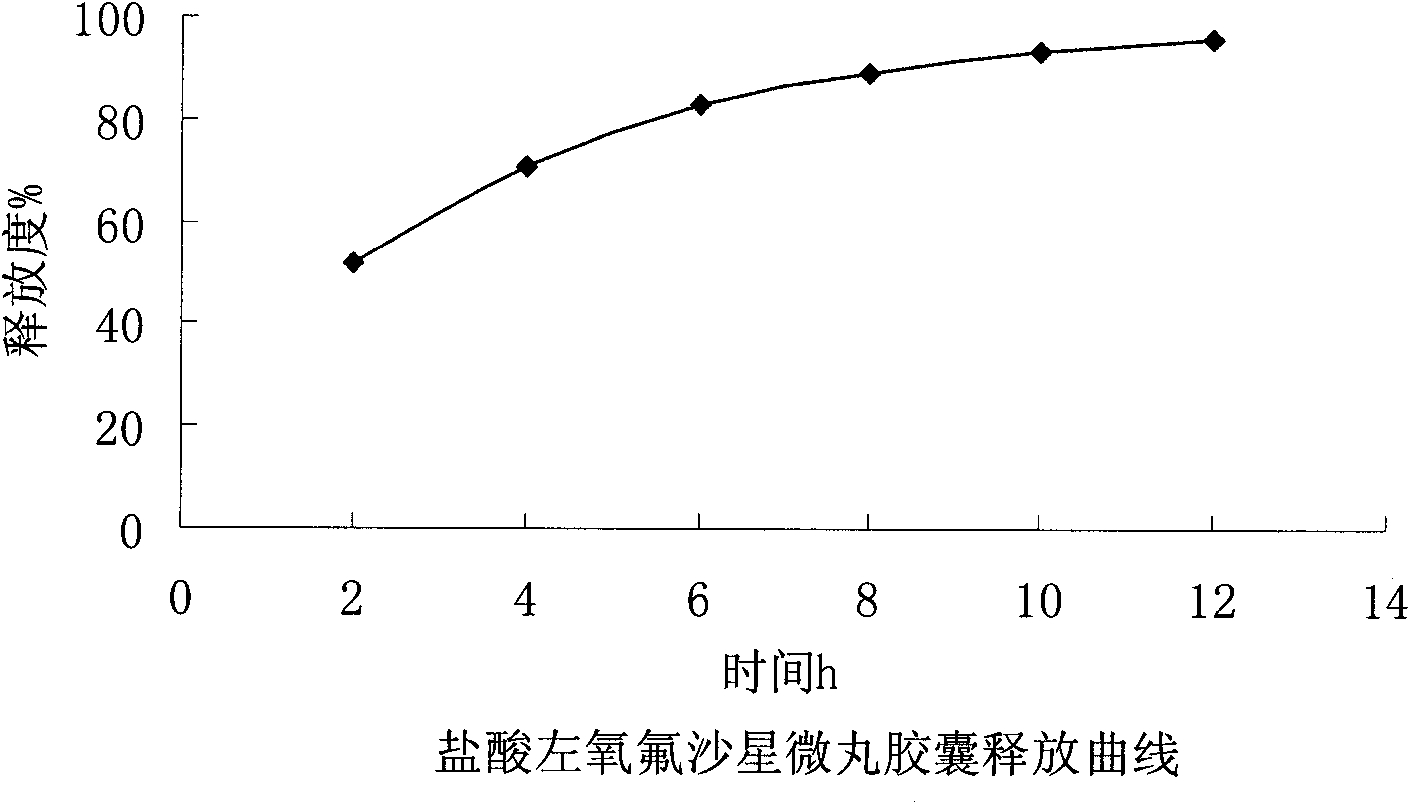
Embodiment 2
[0049] Embodiment 2: the present embodiment adopts following prescription:
[0050] Levofloxacin hydrochloride (calculated as ofloxacin) 400g
[0051] Sucrose core 300g
[0052] povidone k30 36g
[0053] Eudragit NE30D 122g
[0054] Talc powder 36.8g
[0055] Makes 1000 capsules
[0056] Preparation Process:
[0057] (1) Preparation of pill core:
[0058]First 21.6g of povidone k30 is dissolved in the ethanol solution, 240g of levofloxacin hydrochloride is added in the povidone ethanol solution, and constantly stirred to make it into a uniform suspension; 300g of sucrose ball cores are placed in a fluidized bed In the granulator, the above-mentioned uniform suspension is sprayed on the surface of the blank sucrose ball core to prepare micro-pills. After all the suspension is added, dry and screen to obtain micro-pills;
[0059] (2) Sustained-release coating layer:
[0060] Dissolve the Eudragit NE30D and talcum powder of the prescribed amount in water, and stir to obta...
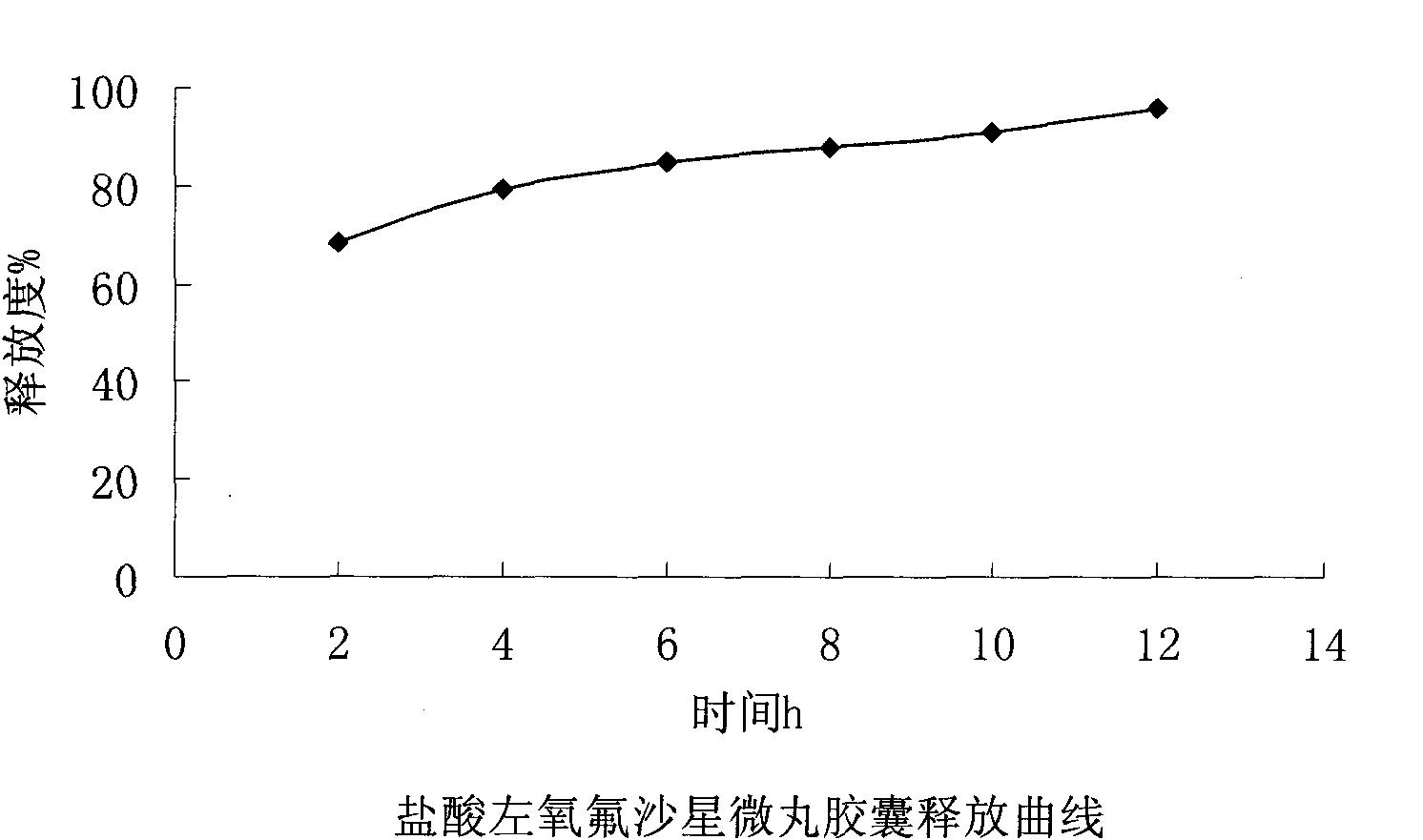
Embodiment 3
[0064] Embodiment 3: the present embodiment adopts following prescription:
[0065] Levofloxacin hydrochloride (calculated as ofloxacin) 100g
[0067] Hypromellose 20g
[0068] Ethylcellulose 8g
[0069] Triethyl citrate 1.6g
[0070] Macrogol 6000 2.4g
[0071] Appropriate amount of talcum powder
[0072] Makes 1000 capsules
[0073] Preparation Process:
[0074] (1) Preparation of pill core:
[0075] First, 8g of hydroxypropylmethylcellulose was dissolved in the ethanol solution, and 40g of levofloxacin hydrochloride was added to the povidone ethanol solution, and kept stirring to make it a uniform suspension; 80g of sucrose ball cores were placed in a fluidized bed In the granulator, the above-mentioned homogeneous suspension is sprayed on the surface of the blank sucrose pellet core to prepare pellets, and after all the suspension is added, the pellets are dried and screened;
[0076] (2) Sustained-release coating layer:
[0077] Diss...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com