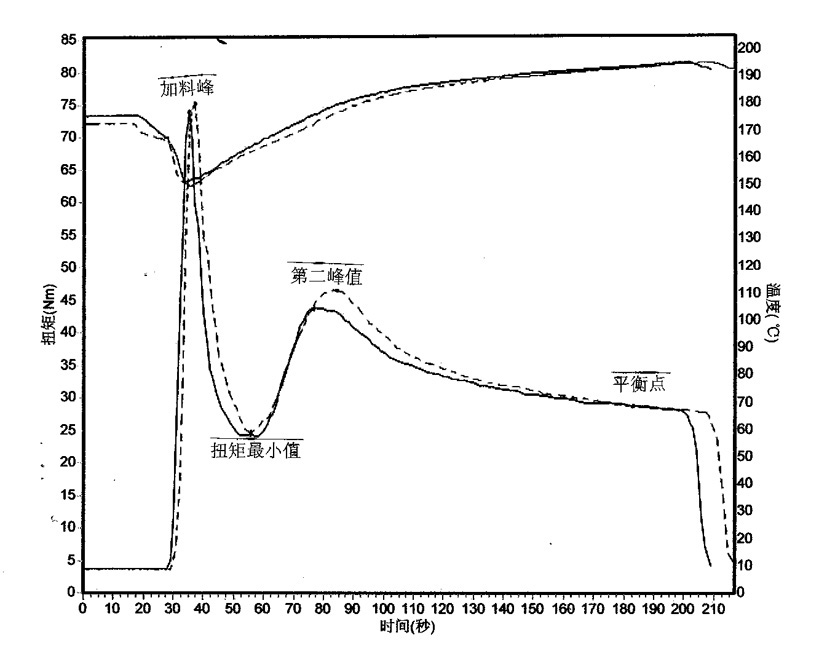
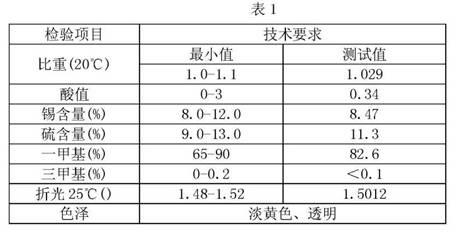
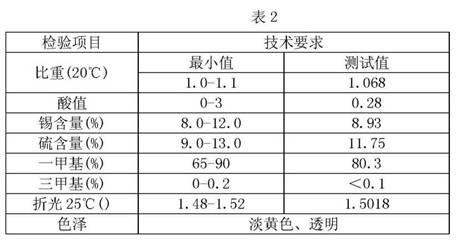
Preparation method of methyl tin heat stabilizer
A technology of methyl tin heat and stabilizer, applied in tin organic compounds and other directions, can solve the problems of large dosage and high production cost, and achieve the effects of reducing formula cost, small addition amount and excellent thermal stabilization effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Preparation of methyl tin chloride intermediate
[0025] Reaction formula:
[0026]3Sn + 6CH3Cl = CH3SnCl3 + (CH3) 2SnCl2 + (CH3) 3SnCl
[0027] (CH3) 3SnCl + SnCl4 = CH3SnCl3 + (CH3) 2SnCl2
[0028] The overall reaction formula:
[0029] Catalyst (CH3)4NCl
[0030] Sn + CH3Cl + SnCl4 → (CH3)xSnCly
[0031] The equipment used in this example: reaction kettle (with stirring system), still (with stirring system), distillation absorption device, metering tank.
[0032] 1. Alkyl halogenation reaction: Check the reaction kettle, transmission device, instrument, pipeline, and absorption device to make them in a normal state. Heat the reaction kettle to raise the temperature of the kettle to 120°C, turn on the vacuum system to make the inside of the kettle in a state of slight negative pressure, open the feeding port of the reaction kettle and add metal tin and tetramethylammonium chloride, close the feeding port, and feed in methyl chloride, Make the pressu...
Embodiment 2
[0035] Example 2: Preparation of methyl tin chloride intermediate
[0036] The specific scheme is the same as that in Example 1, except that the amount of each substance added is: metal tin (Sn) 80 kg, methyl chloride (CH3Cl) 95 kg, tetramethylammonium chloride ((CH3)4NCl) 3.2 kg, tetrachloromethane Tin (SnCl4) 120 kg, water 300 kg. The reaction time was 7 hours. During the distillation process, the kettle temperature was controlled at 150-160° C., and the entire distillation process took 1.5 hours. Measured to obtain 558.8 kg of an intermediate aqueous solution with a mass concentration of 46.31%, with a yield of 96.62%. The aqueous solution is colorless and transparent.
Embodiment 3
[0037] Example 3: Preparation of methyl tin chloride intermediate
[0038] The specific scheme is the same as in Example 1, the difference is that the amount of each substance added is: metal tin (Sn) 95 kg, methyl chloride (CH3Cl) 102 kg, tetramethylammonium chloride ((CH3)4NCl) 3.2 kg, tetramethylammonium chloride ((CH3)4NCl) 3.2 kg, Tin chloride (SnCl4) 128 kg. 300 kg of water. During the distillation process, the kettle temperature was controlled at 170-180° C., and the entire distillation process took 1 hour. Measured to obtain 594.5 kg of an intermediate aqueous solution with a mass concentration of 49.54%, the yield was 97.0%, and the aqueous solution was colorless and transparent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com