Process for recovering sulfuric acid and sulfate from waste acid generated in preparation of titanium dioxide by using sulfuric acid method
A sulfate, titanium dioxide technology, applied in the direction of ferric sulfate, sulfur trioxide/sulfuric acid, sulfur compounds, etc., to achieve the effect of high recycling rate, strong practicability, good acid and corrosion resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The waste titanium dioxide acid sampled by a company was analyzed to be composed of 20.01% sulfuric acid, 15.72% ferrous sulfate, 62.84% water, and a small amount of other impurities. Utilize the method of the invention to process the waste titanium dioxide acid of this company.
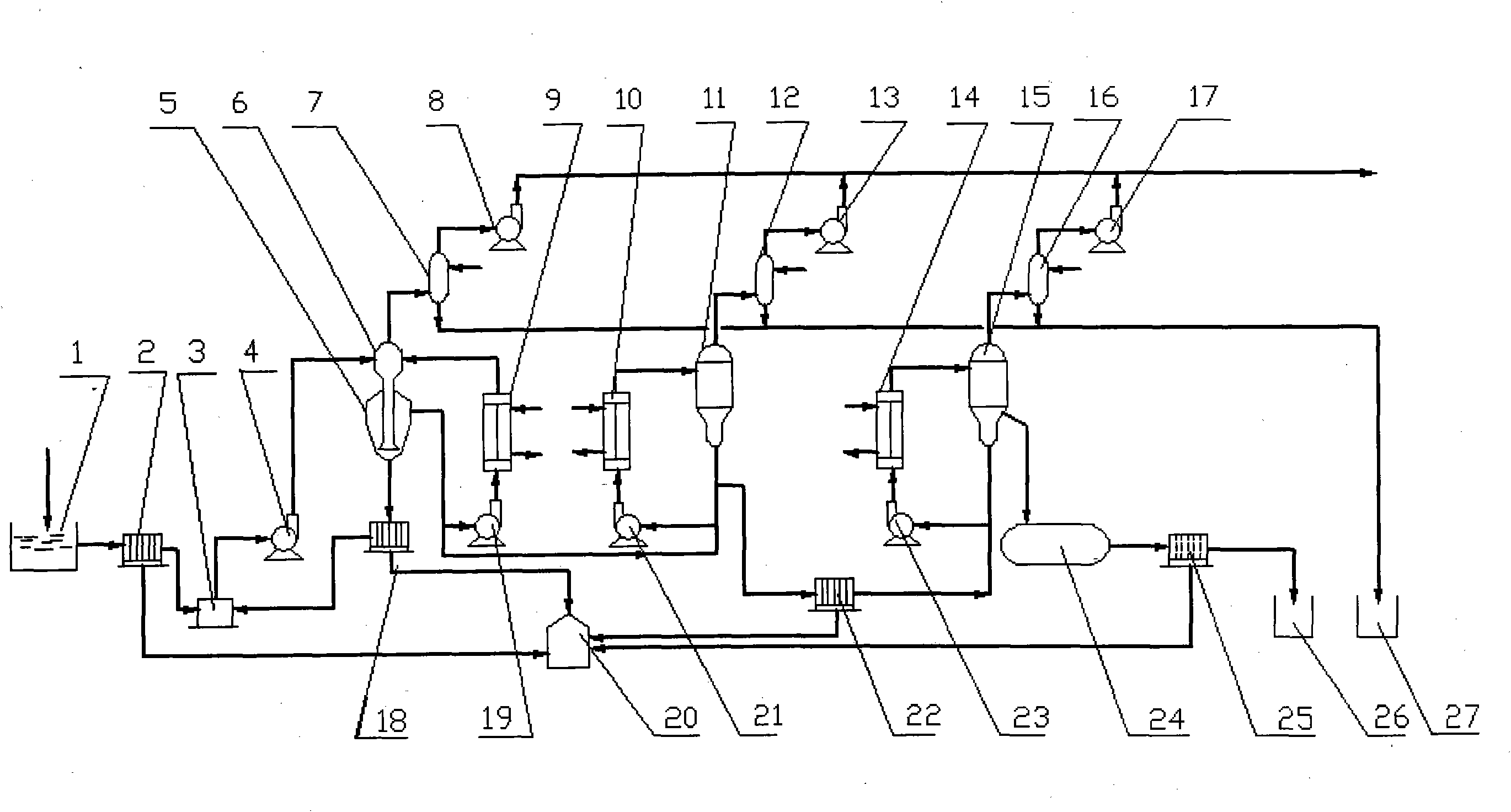
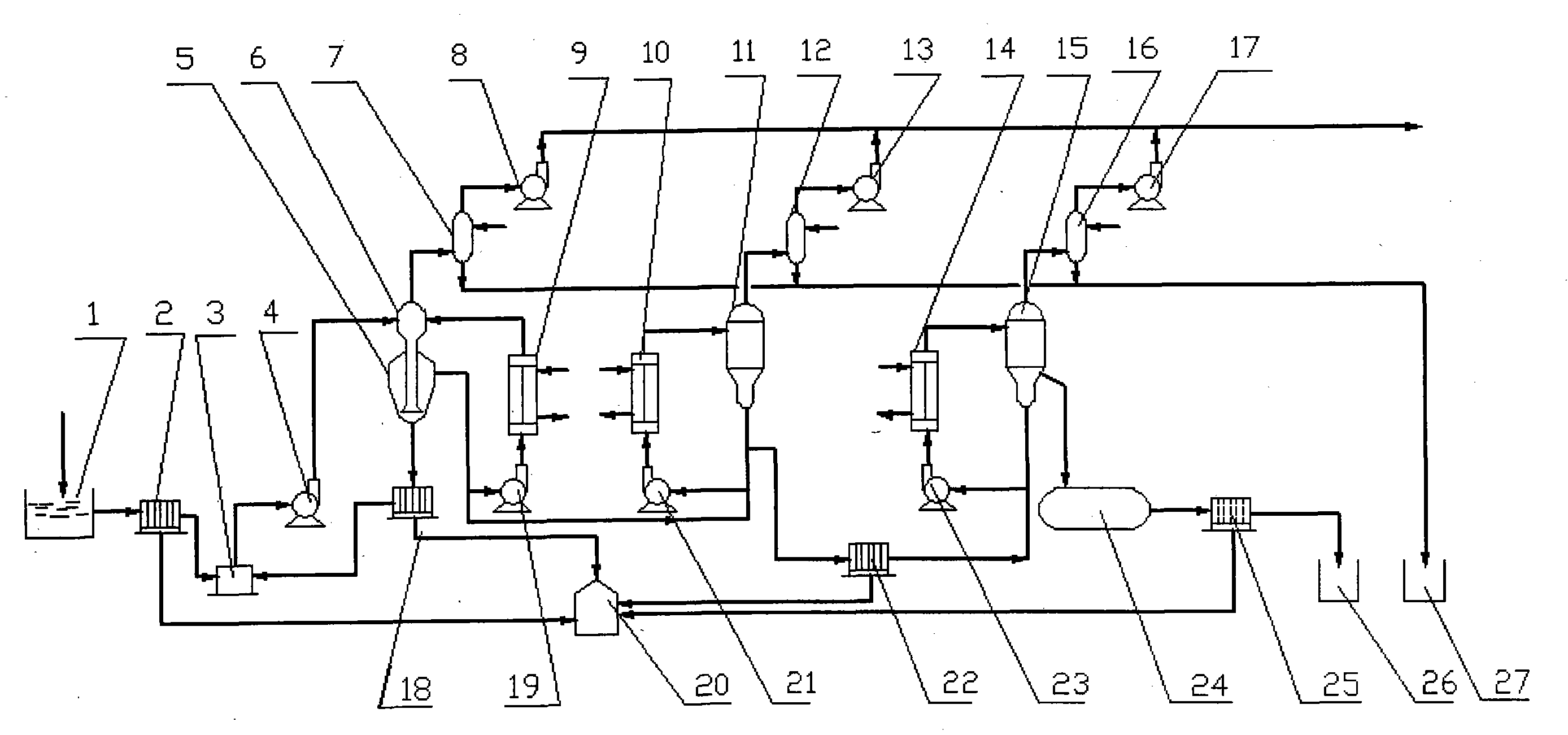
[0026] like figure 1 Shown, a kind of process of reclaiming sulfuric acid and vitriol from the waste acid that sulfuric acid method prepares titanium dioxide, its concrete steps are as follows:
[0027] (1) Precipitation pretreatment
[0028] The waste acid produced in the first washing process of preparing titanium dioxide by the sulfuric acid method is first sent to the inclined plate settling tank 1 through the pipeline, and the precipitation pretreatment is carried out to separate the coarse-grained sulfate. The coarse-grained sulfate precipitated in the inclined plate settling tank 1 is recovered and reused; the supernatant of the inclined plate settling tank 1 is transported to the dia...
Embodiment 2
[0036] A kind of specific steps of the process of reclaiming sulfuric acid and sulfate from the waste acid of titanium dioxide prepared by sulfuric acid method, same as implementation case 1, wherein:
[0037] In the (2) step, the absolute pressure of flash evaporation in the evaporation chamber 6 of the vacuum crystallizer is 35KPa, and crystallization is carried out at a temperature of 85° C. in the crystallization chamber 5 of the vacuum crystallizer. The first heater 9 is heated with steam at a temperature of 135°C, and the temperature of the circulating fluid reaches 120°C. After sampling and analysis, the concentration of sulfuric acid in the primary concentrated acid is 37.6%, and the amount of ferrous sulfate in the primary concentrated acid is 5.83%.
[0038] In step (3), the second heater 10 is heated with steam at a temperature of 145° C. to heat the temperature of the circulating acid to 140° C. The absolute pressure of vacuum evaporation in the second-stage vacuu...
Embodiment 3
[0041] A kind of specific steps of the process of reclaiming sulfuric acid and sulfate from the waste acid of titanium dioxide prepared by sulfuric acid method, same as implementation case 1, wherein:
[0042] In the (2) step, the absolute pressure of flash evaporation in the evaporation chamber 6 of the vacuum crystallizer is 27.5KPa, and crystallization is carried out at a temperature of 76° C. in the crystallization chamber 5 of the vacuum crystallizer. The first circulating pump 19 sends the first heater 9 to heat with steam at a temperature of 130° C., and the temperature of the circulating fluid reaches 118° C. After sampling and analysis, the concentration of sulfuric acid in the primary concentrated acid is 34.8%, and the amount of ferrous sulfate in the primary concentrated acid is 6.89%.
[0043] In step (3), the second heater 10 is heated with steam at a temperature of 135° C. to heat the temperature of the circulating acid to 130° C. The absolute pressure of vacuu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com