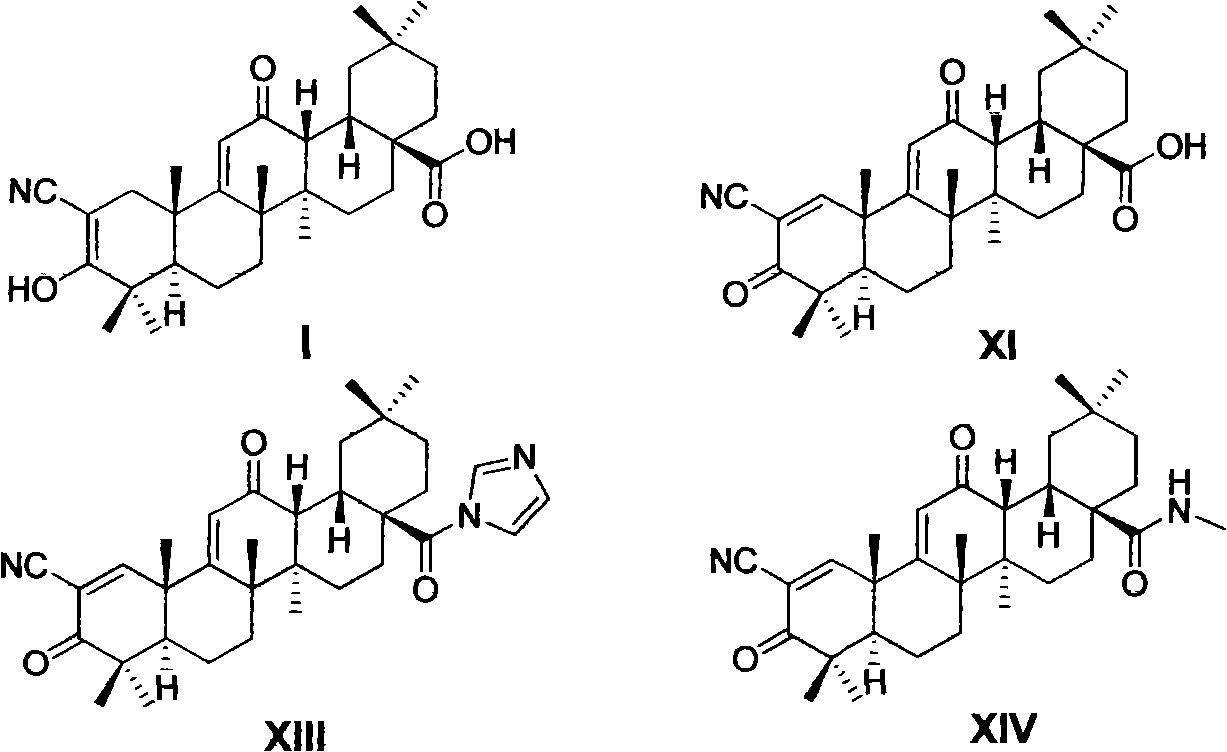
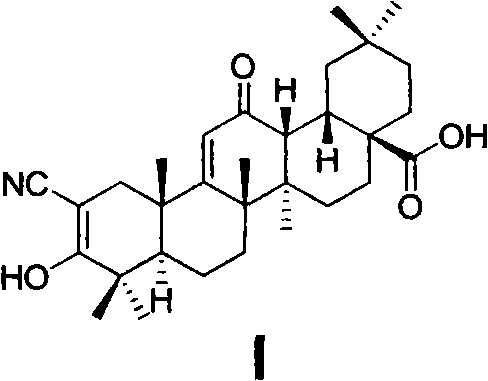
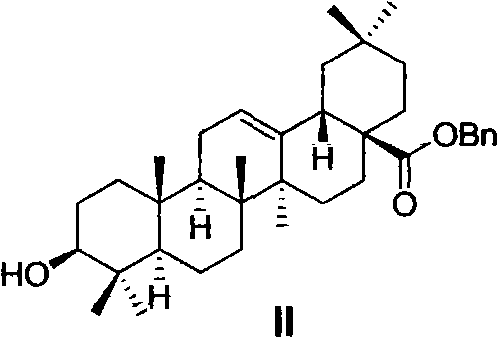
Oleanolic acid derivative, and preparation method and purpose thereof
A technology for oleanolic acid derivatives, applied in the field of preparation of oleanolic acid derivatives or pharmaceutically acceptable salts thereof, can solve the problems of low reactivity, high price, inflammability and explosion, and achieve The effect of high reaction yield, low production cost and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Preparation of Benzyl 3β-Hydroxyoleanane-12-ene-28-carboxylate (II)
[0056] Oleanolic acid (100g, 220mmol) and K 2 CO 3 (61g, 440mmol) was placed in DMF (800mL), and benzyl chloride (33mL, 290mmol) was added dropwise within 20 minutes at 50-55°C. After dropping, the reaction was continued for 3-4 hours, cooled to room temperature, filtered, and the filter cake was Washed with DMF (50mL×3), the filtrate was poured into ice water (3000mL), a large amount of white solids were precipitated, left to stand until the solid particles became larger, filtered with suction, the solids were collected, fully washed with water, and dried to obtain a white solid II (114g , 95.5%). Compound II is a known compound with a CAS number of 303114-51-4.
Embodiment 2
[0058] Preparation of Benzyl 3β-Acetoxyoleanane-12-ene-28-carboxylate (III)
[0059] Compound II (5.46g, 10mmol) was dissolved in 20mL of pyridine, and acetic anhydride (10.2g, 100mmol) was slowly added dropwise at 0-5°C. After the addition was completed, DMAP (0.12g, 1mmol) was added, and a solid precipitated out. Continue to react for 1 to 2 hours, add an appropriate amount of dichloromethane (50mL) to dissolve, wash the solution with 5% HCl solution, saturated sodium bicarbonate solution, and saturated brine for 3 times, dry over anhydrous sodium sulfate, and remove the solvent under reduced pressure A white solid III (5.4 g, 91.3%) was obtained. Compound III is a known compound with a CAS number of 357953-27-6.
Embodiment 3
[0061] Preparation of Benzyl 3β-Acetoxy-12-oxooleanane-28-carboxylate (IV)
[0062] Compound III (5.88g, 10mmol) was dissolved in an appropriate amount of dichloromethane (50mL), added formic acid (10mL), H 2 o 2 (1.36g, 40mmol), reacted at room temperature for 24 hours, TLC monitored the reaction process, after the disappearance of the raw material point, the reaction solution was washed to near neutrality with saturated sodium bicarbonate solution, washed 3 times with saturated saline, dried over anhydrous sodium sulfate, After removing the solvent under reduced pressure, a light yellow solid was obtained, AcOH-H 2 O was recrystallized to give IV as a white solid (4.7 g, 78%). Compound IV is a known compound with CAS No. 357953-28-7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com