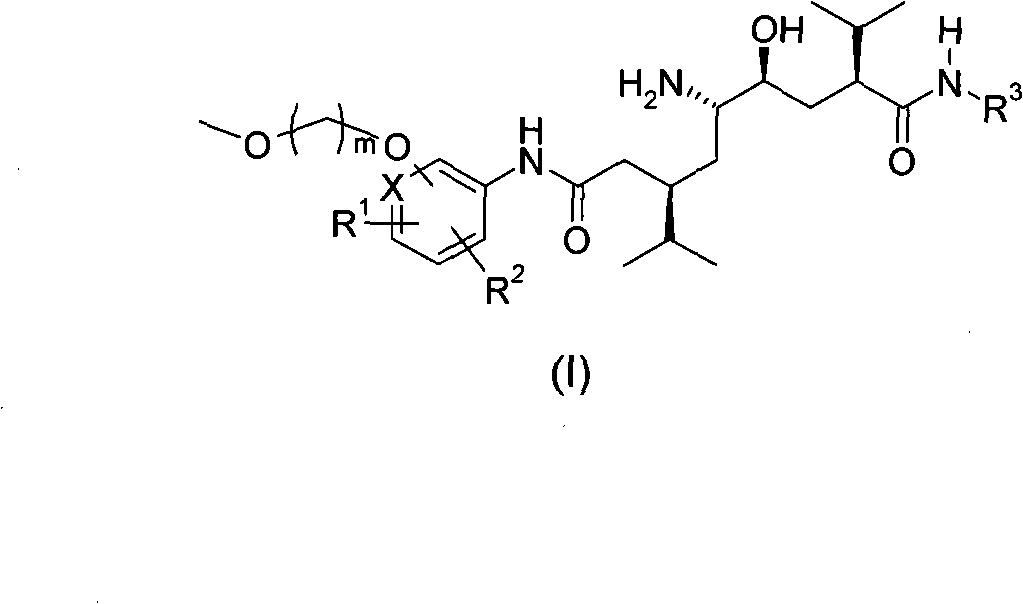
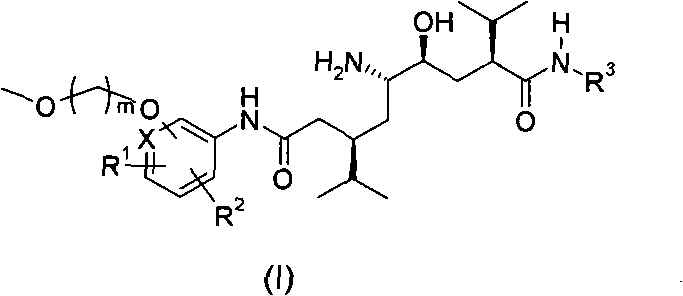
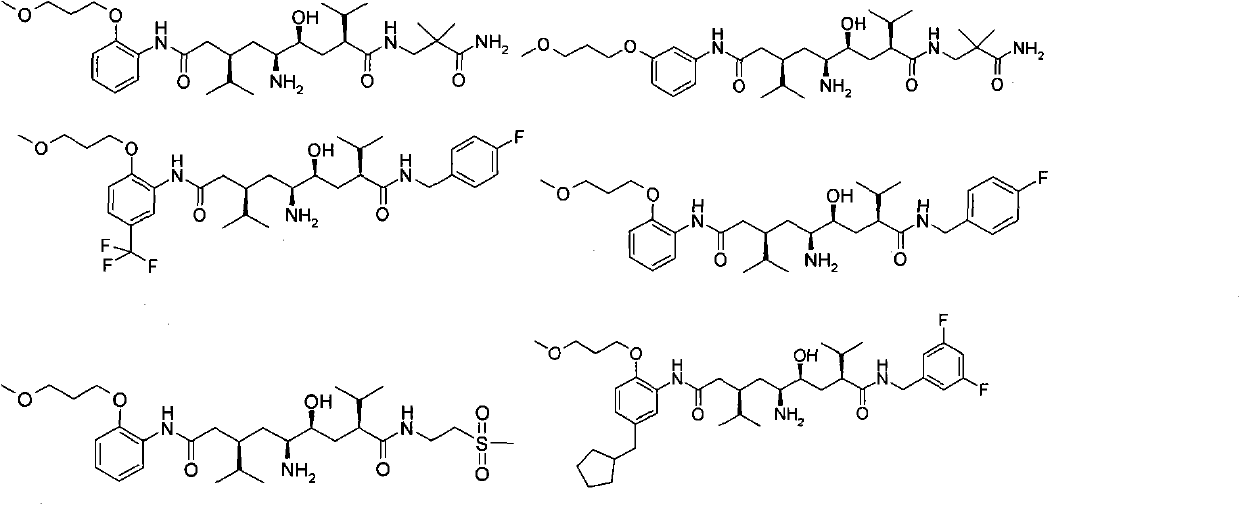
5-amino-4-hydroxy-N-aryl azelamide derivatives as well as preparation methods and medical applications thereof
An alkyl and compound technology, which is applied to 5-amino-4-hydroxy-N-aryl azelaic amide derivatives, their preparation and their application in medicine, can solve the problems of low human bioavailability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0156] (2S, 4S, 5S, 7R)-5-amino-N 1 -(3-amino-2,2-dimethyl-3-oxopropyl)-4-hydroxy-2,7-diisopropyl-N 9 -(2-(3-Methoxypropoxy)phenyl)azalamide
[0157]
[0158]
[0159] first step
[0160] 3-Methoxypropyl 4-toluenesulfonate
[0161] Under ice-cooling, 3-methoxy-1-propanol 1a (45.01g, 0.50mol) was dissolved in 200mL of dichloromethane and triethylamine (252.50g, 2.50mol) mixed solvent, p-toluenesulfonyl chloride ( 104.80g, 0.55mol), stirred at room temperature for 3 hours. Add 200mL water to the reaction solution, extract with dichloromethane (150mL×3), combine the organic phases, and successively wash with saturated sodium bicarbonate solution (50mL×2), saturated ammonium chloride solution (50mL×2) and saturated saline Wash (50mL×2), dry over anhydrous sodium sulfate, filter, concentrate the filtrate under reduced pressure, and purify the residue by silica gel column chromatography (eluent: system B in appropriate proportion) to obtain the title product 3-methoxy Pro...
Embodiment 2
[0193] (2S, 4S, 5S, 7R)-5-amino-N 1 -(3-amino-2,2-dimethyl-3-oxopropyl)-4-hydroxy-2,7-diisopropyl-N 9 -(3-(3-Methoxypropoxy)phenyl)azelamide
[0194]
[0195] first step
[0196] 1-(3-Methoxypropoxy)-3-nitrobenzene
[0197] 3-Nitrophenol (2.08g, 15.01mmol) was dissolved in 30mL of acetonitrile, 3-methoxypropyl 4-tosylate 1b (4.03g, 16.50mmol) and anhydrous potassium carbonate (6.21g, 45.01 mmol), stirred and reacted at 70°C for 12 hours. Filter, add 150mL ethyl acetate to the filtrate, wash with saturated sodium bicarbonate solution (50mL×2), saturated ammonium chloride solution (50mL×2) and saturated brine (50mL×2) successively, wash with anhydrous sodium sulfate Dry, filter, and concentrate the filtrate under reduced pressure to obtain the crude product of the title product 1-(3-methoxypropoxy)-3-nitrobenzene 2a (3.17 g, yellow oil).
[0198] second step
[0199] 3-(3-Methoxy-propoxy)aniline
[0200] Dissolve 1-(3-methoxypropoxy)-3-nitrobenzene 2a (3.17g, 15.02mmol...
Embodiment 3
[0217] (2S, 4S, 5S, 7R)-5-amino-N 1 -(4-fluorobenzyl)-4-hydroxy-2,7-diisopropyl-N 9 -(2-(3-Methoxypropoxy)-5-(trifluoromethyl)phenyl)azelaamide
[0218]
[0219] first step
[0220] 1-(3-Methoxypropoxy)-2-nitro-4-(trifluoromethyl)benzene
[0221] 2-Nitro-4-(trifluoromethyl)phenol (0.63 g, 3.01 mmol) was dissolved in 20 mL of tetrahydrofuran, 3-methoxy-1-propanol 1a (308 mg, 3.42 mmol) and triphenyl Phosphine (927mg, 3.54mmol) was added to diethyl azodicarboxylate (783mg, 4.50mmol), and the reaction was stirred for 4 hours. The reaction solution was concentrated under reduced pressure, and the resulting residue was purified by silica gel column chromatography (eluent: appropriate proportion of system B) to obtain the title product 1-(3-methoxypropoxy)-2-nitro-4 -(Trifluoromethyl)benzene 3a (800 mg, yellow oil), yield 95.6%.
[0222] second step
[0223] 2-(3-Methoxypropoxy)-5-(trifluoromethyl)aniline
[0224] 1-(3-Methoxypropoxy)-2-nitro-4-(trifluoromethyl)benzene 3a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com