Hardenable compositions based on silylated polyurethanes
A technology of polymers and compounds, applied in polyurea/polyurethane adhesives, adhesive types, adhesives, etc., can solve problems such as unsatisfactory mechanical properties of adhesives and sealants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0099] polymer manufacturing
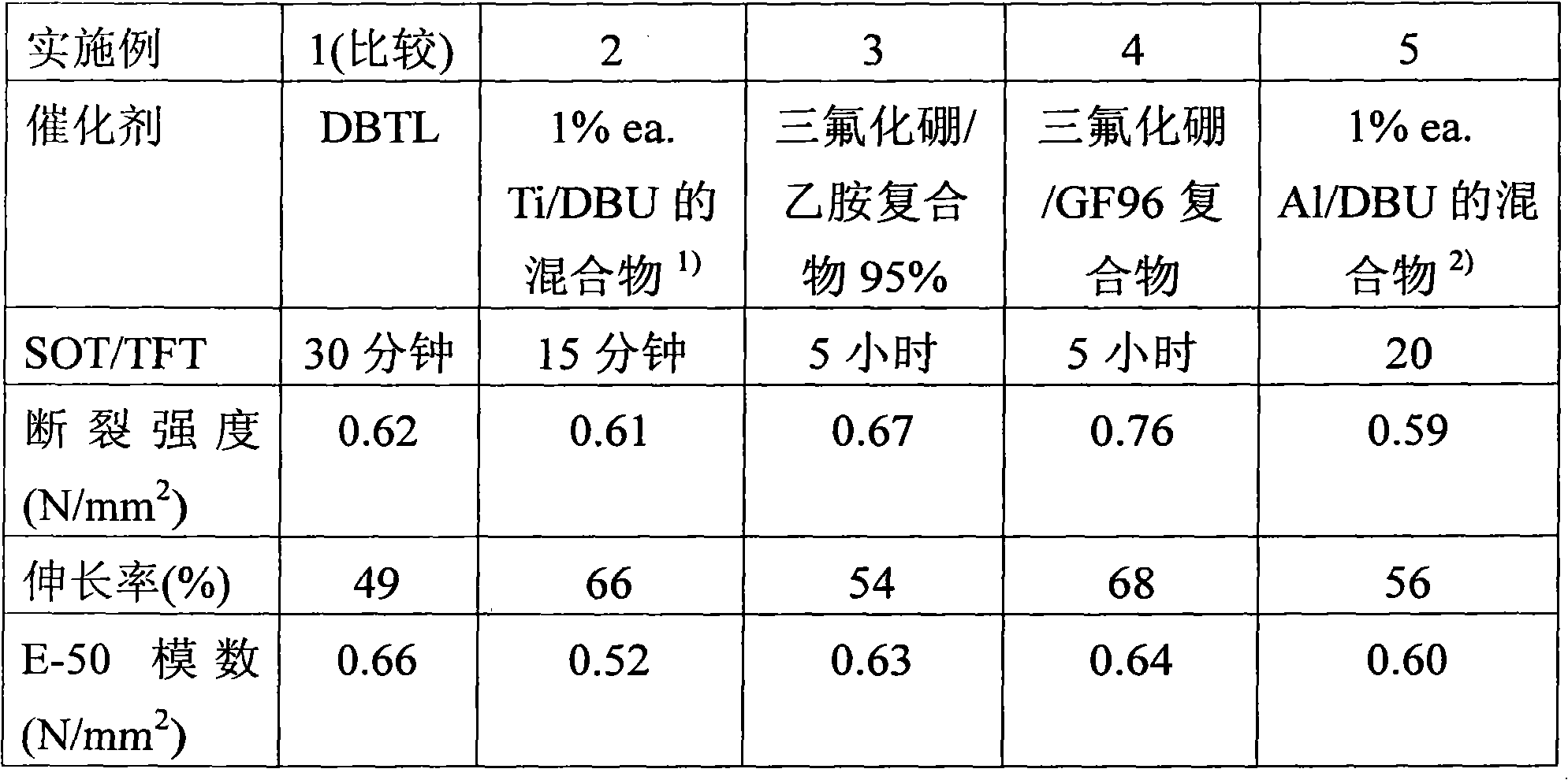
[0100] 282 g (15 mmol) of polypropylene glycol 18000 (OH number=6.0) were dried in a 500 ml three-necked flask under vacuum at 100°C. Under a nitrogen atmosphere at 80°C, 0.1 g of bismuth carboxylate (Borchi Kat 24, Borchers co.) was added, followed by 7.2 g (32 mmol) of 3-isocyanatopropyltrimethoxysilane (%NCO=18.4). After stirring at 80° C. for 1 hour, the polymer obtained was cooled and 6 g of vinyltrimethoxysilane were added.
[0101] Properties of polymer films
[0102] In an aluminum pan with a diameter of 50 mm, 5 g of prepolymer were mixed with 0.05 g of AMMO and 0.05 g of A1170, and 0.025 g of the corresponding catalyst. The surface curing time (skin-over time, SOT) and the time until the formation of a tack-free layer (tack-free time, TFT) were determined for these mixtures (in each case at 23° C. and 50% relative humidity). In addition, the aforementioned mixture was coated with a layer thickness of 2 mm on a glass plate on which a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com