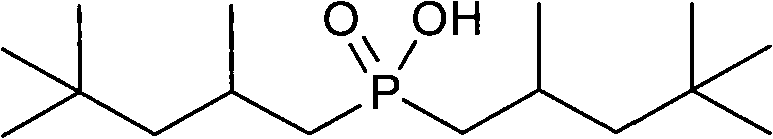
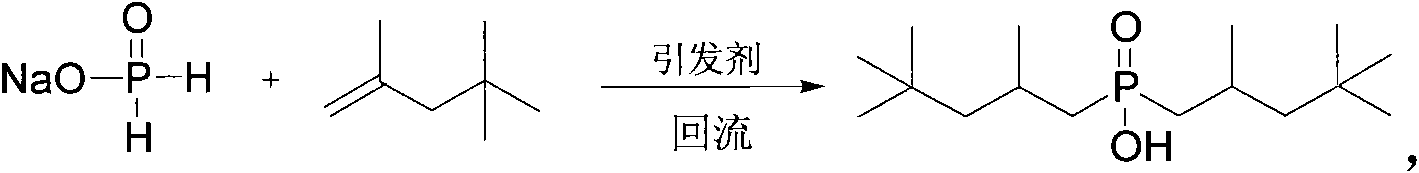
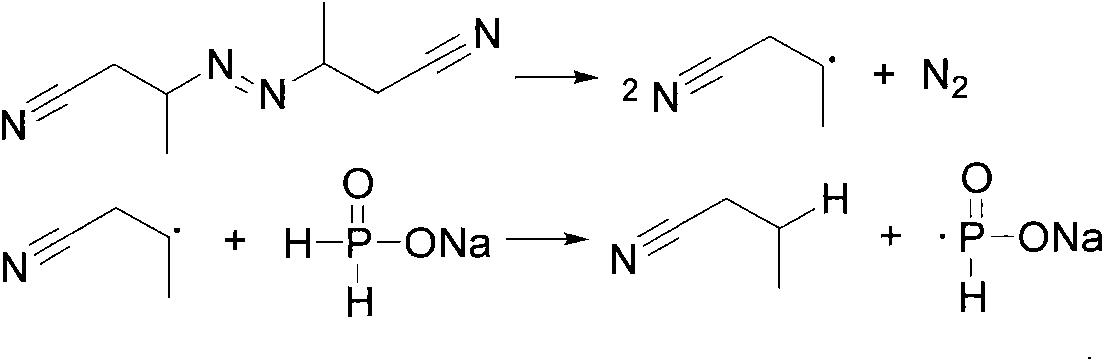
Method for synthesizing bis(2,4,4-trimethylpentyl) phosphinic acid under normal pressure
A technique for synthesizing trimethylpentyl under normal pressure, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve harsh reaction conditions, high equipment requirements, and long reaction time problems such as mild reaction conditions, shortened reaction time, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 50.0g (471mmol) of sodium hypophosphite, 50.0g (833mmol) of acetic acid and 211.40g (1413mmol) of diisobutylene (75%) in a 1000mL four-necked flask equipped with a mechanical stirrer, a thermometer, a condenser and a dropping funnel. , stirred, and dropwise added 3.09g (18.84mmol) of benzene solution of azobisisobutyronitrile from the dropping funnel at room temperature. The benzene solution of g (9.42mmol) azobisisobutyronitrile was added with an initiator every 2 hours. After reacting for 10 hours, the temperature was lowered to 50° C., washed with alkali, acidified, dried and vacuum rotary evaporated to obtain the product di(2,4,4-trimethylpentyl)phosphinic acid. The composition of the product is as follows:
[0023] 31 P-NMR analysis:
[0024] Target product di(2,4,4-trimethylpentyl)phosphinic acid 78%
[0025] Mono-substituted 2,4,4-trimethylpentylphosphonous acid 13.8%
[0026] Other components 8.2%.
Embodiment 2
[0028] According to the method of Example 1, the time interval for adding the initiator was changed to 1 hour, and the other methods were unchanged. The composition of the product is as follows:
[0029] 31 P-NMR analysis:
[0030] Target product bis(2,4,4-trimethylpentyl)phosphinic acid 88.9%
[0031] Mono-substituted 2,4,4-trimethylpentylphosphonous acid 6.4%
[0032] Other components 4.7%.
Embodiment 3
[0034] According to the method of Example 1, the time interval for adding the initiator was changed to 3 hours, and the other methods were unchanged. The composition of the product is as follows:
[0035] 31 P-NMR analysis:
[0036] Target product di(2,4,4-trimethylpentyl)phosphinic acid 71.4%
[0037] Mono-substituted 2,4,4-trimethylpentylphosphonous acid 17.5%
[0038] Other components 11.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com