Proline ionic liquid and preparation method and application thereof
A technology of ionic liquid and proline, which is applied in the preparation of carboxylic acid nitriles, organic chemical methods, chemical instruments and methods, etc., can solve the problems of difficult to remove, cumbersome operation process, etc., and achieve simple separation and simple preparation process , fast response effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) N-methylimidazole and chlorobutane are passed through a quaternization reaction in a molar ratio of 1: 1.1, the reaction temperature of the quaternization reaction is 70° C., and the reaction time is 48 hours to obtain 1-butyl- Chloride salt of 3-methylimidazole, yield 93.7%;
[0031] (2) Proline and sodium hydroxide were neutralized at room temperature with a molar ratio of 1:1 for 2 hours to obtain proline sodium salt; proline sodium salt was dissolved in methanol to obtain proline sodium methanol solution;
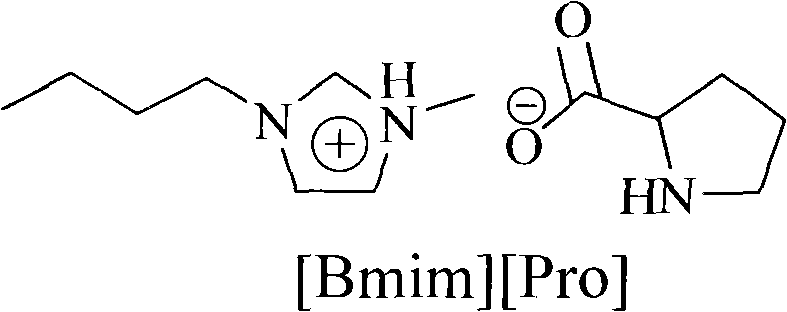
[0032] (3) the methanol solution of sodium proline obtained in step (2) and the chloride salt of 1-butyl-3-methylimidazole obtained in step (1) are in a molar ratio of 1: 0.9 at room temperature for anion exchange, time After 12 hours, filter, remove the solvent, and vacuum-dry to obtain the proline ionic liquid [Bmim][Pro], the yield is 91.1%. The structure of proline ionic liquid [Bmim][Pro] is as follows:
[0033]
Embodiment 2
[0035] (1) Pyridine and ethyl bromide are passed through a quaternization reaction in a molar ratio of 1: 1.4, the reaction temperature of the quaternization reaction is 100° C., and the reaction time is 24 hours to obtain 1-ethylpyridinium bromide with a yield of 89.7 %;
[0036] (2) neutralize L-proline and sodium hydroxide at room temperature with a molar ratio of 1:1 for 0.5 hours to obtain proline sodium salt; dissolve proline sodium salt in methanol to obtain proline Methanol solution of sodium;
[0037] (3) the methanol solution of sodium proline obtained in step (2) and the 1-ethylpyridinium bromide salt obtained in step (1) are in a molar ratio of 1: 0.7 at room temperature for anion exchange, and the time is 18 hours, filtered, The solvent was removed and the proline ionic liquid was obtained after vacuum drying with a yield of 88.1%.
Embodiment 3
[0039] (1) Pyrrole and hexyl bromide are passed through a quaternization reaction in a molar ratio of 1: 1.3, the reaction temperature of the quaternization reaction is 60° C., and the reaction time is 72 hours to obtain 1-ethylpyrrole bromide with a yield of 91.2 %;
[0040] (2) neutralize L-proline and sodium hydroxide at room temperature with a molar ratio of 1:1 for 3 hours to obtain proline sodium salt; dissolve proline sodium salt in methanol to obtain proline Methanol solution of sodium;
[0041] (3) the methanol solution of sodium proline obtained in step (2) and the 1-ethylpyrrole bromide salt obtained in step (1) are in a molar ratio of 1: 0.8 for anion exchange at room temperature, and the time is 24 hours, filtered, The solvent was removed and the proline ionic liquid was obtained after vacuum drying with a yield of 90.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com