Anti-tumor natural medicine coupled with nitric oxide donor and medical use thereof
A technology of nitric oxide and natural medicine, applied in the field of medicine for diseases, can solve problems such as insufficient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
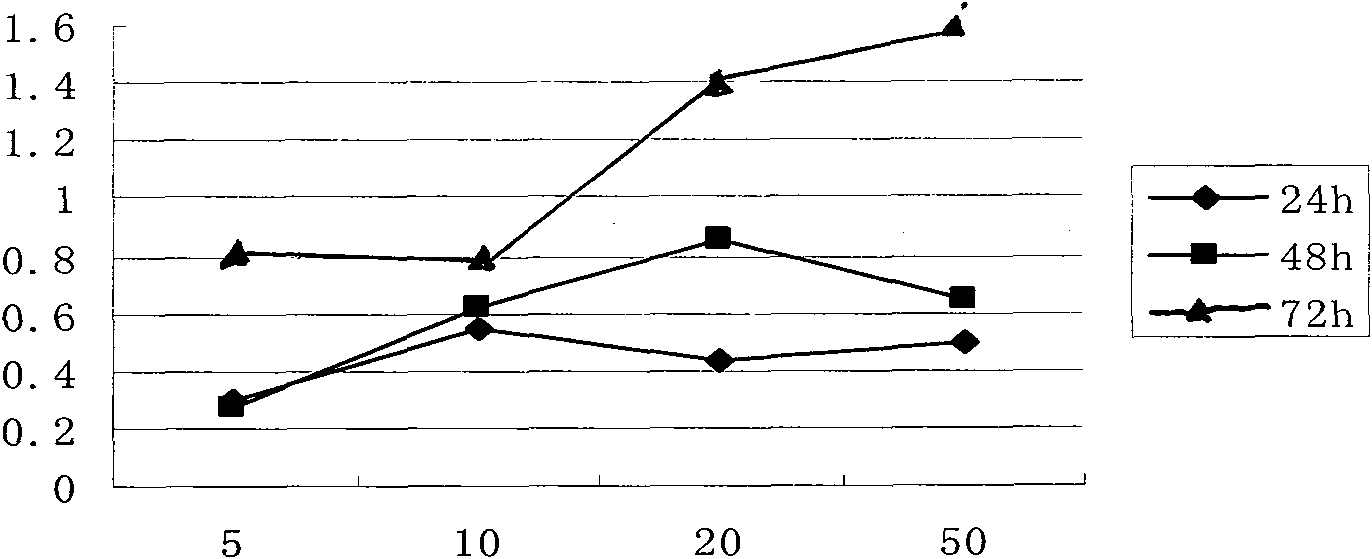
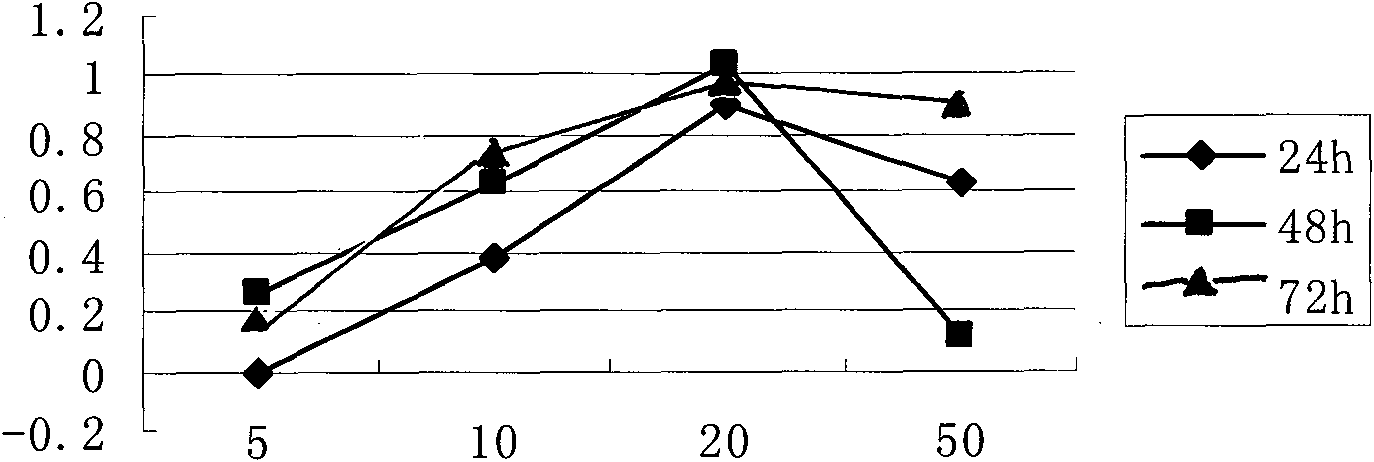
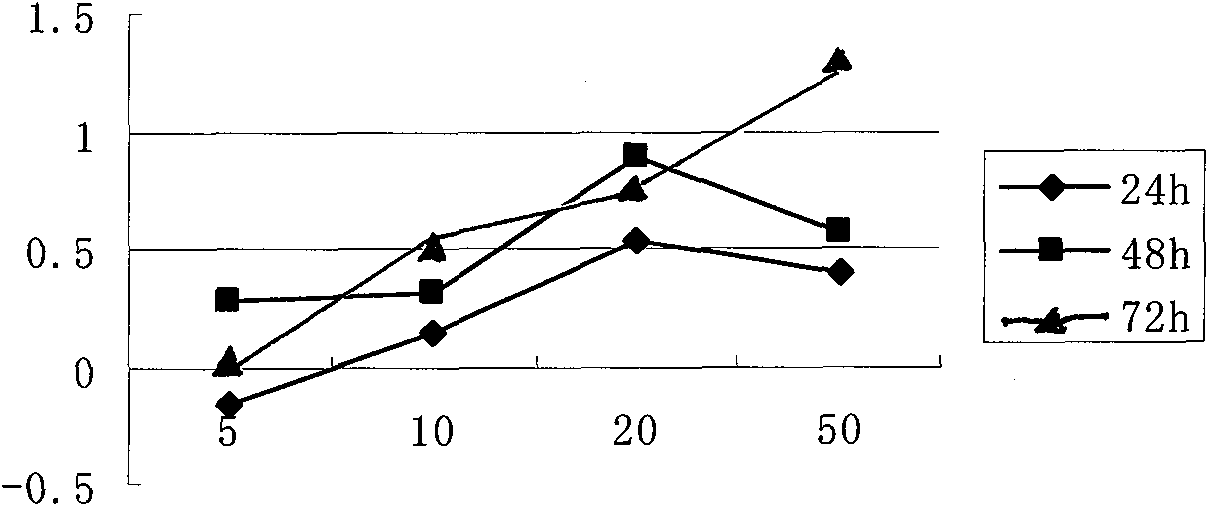
Image
Examples
Embodiment 1
[0090] Example 1: Rhein and Nitric Oxide Donor Coupling Compound I
[0091] The synthetic equation of this coupling compound I is:
[0092]
[0093] The chemical name of this coupling compound I:
[0094] 4-(2-(4,5-dihydroxy-9,10-dioxo-9,10-dihydroanthracene-2-carbonyloxy)ethoxy)-3-(phenylsulfonyl)-1,2,5-oxadiazole 2-oxide.
[0095] The preparation process of this coupling compound I is:
[0096] The first step: slowly add NaOH (70mmol) with a mass ratio of 50% to compound 2 (chemical name: 3,4-bis(phenylsulfonyl)-1,2,5-oxadiazole 2-oxide, 70mmol) and bromine Ethanol (70mmol) in THF (400ml) solution, the resulting mixture was stirred at room temperature for 16 hours, then 50% NaOH (20mmol) was added dropwise and stirred for 2 hours to complete the reaction. The organic solvent was evaporated and the remaining material was poured into ice water. The product was repeatedly extracted three times with dichloromethane (100 ml), the organic layers were combined, dried over an...
Embodiment 2
[0101] Example 2: Rhein and Nitric Oxide Donor Coupling Compound II
[0102] The synthetic equation of this coupling compound II is:
[0103]
[0104] The chemical name of the coupling compound II:
[0105] (Z)-2-((4,5-dihydroxy-9,10-dioxo-9,10-dihydroanthracene-2-carbonyloxy)methoxy)-1-(pyrrolidin-1-yl)diazene oxide.
[0106] The preparation process of this coupling compound II is:
[0107] Compound 1 (rhein, 14mmol) was dissolved in DMF (100ml), and Na 2 CO 3 (15mmol) and KI (2mmol), after stirring for 15 minutes, add compound 4 (chemical name: (Z)-2-(chloromethoxy)-1-(pyrrolidin-1-yl) diazene oxide, 12mmol). The resulting compound was stirred at 100°C for 16 hours. After cooling, the solid was filtered off, the product was extracted three times with EtOAc (30ml), the organic layers were combined, dried over anhydrous magnesium sulfate, filtered and the solvent was evaporated. Compound II (0.8 g) was purified by silica gel chromatography (Haxane-20% haxane / EtOAc).
...
Embodiment 3
[0113] Example Three: Ursolic Acid and Nitric Oxide Donor Coupling Compound III
[0114] The chemical structure of this coupling compound III is:
[0115]
[0116] The chemical name of this coupling compound III:
[0117] (Z)-2-(((1S, 2R, 6aS, 6bR, 10S, 12aR, 12bR)-10-hydroxy-1, 2, 6a, 6b, 9, 9, 12a-heptamethyl-1, 2, 3, 4, 4a, 5, 6, 6a, 6b, 7, 8, 8a, 9, 10, 11, 12, 12a, 12b, 13, 14b-icosahydropicene-4a-carbonyloxy)methoxy)-1-(pyrrolidin-1- yl) diazene oxide.
[0118] The preparation process of the coupling compound III is the same as that of Example 2, only the natural ingredients have been changed, so it will not be repeated. The prepared coupling compound III (2.0 g) was proved by means of nuclear magnetic resonance, mass spectrometry and elemental analysis, and the coupling compound III did have the elemental composition and molecular structure described in the above molecular formula. The specific parameters are:
[0119] 300mz 1 H NMR (DMSO-d6) δ5.74(s, 2H), 5.24...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com