LID-PEG-PLGA controlled-release nano microsphere and preparation method thereof
A technology of LID-PEG-PLGA and nano-microspheres, which is applied in the field of slow-release microspheres and its preparation, can solve problems such as increasing bioavailability, prolonging the action time of LID, and reducing the number of administrations, so as to increase bioavailability, The effect of reducing the frequency of administration and prolonging the action time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
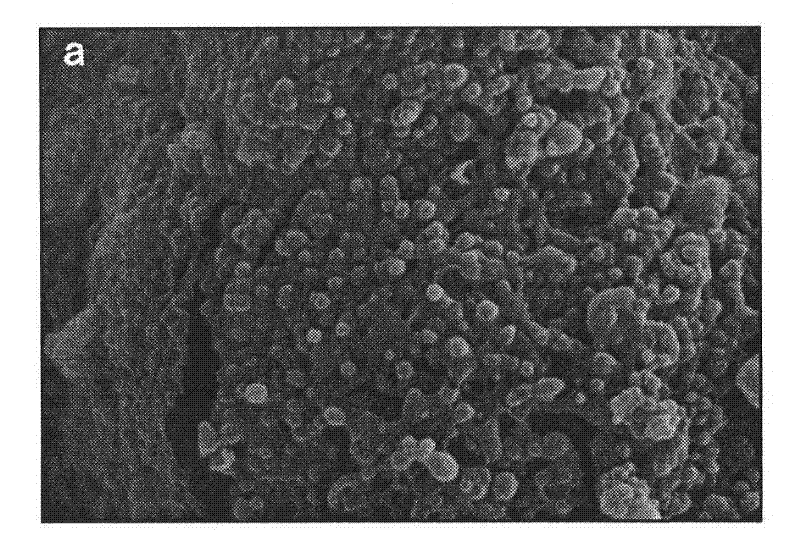
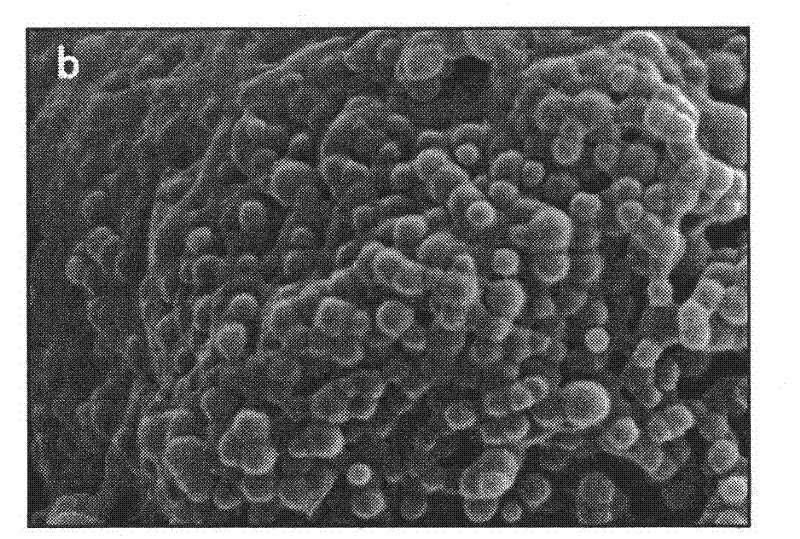
[0024] A LID-PEG-PLGA sustained-release nanometer microsphere is a sustained-release nanometer microsphere containing lidocaine and a degradable carrier, and the degradable carrier contains a lactic acid-glycolic acid copolymer. In the present invention, there is also polyethylene glycol in the degradable carrier, and the mass percentage of the polyethylene glycol and lactic acid-glycolic acid copolymer is 1:20 to 1:10; The mass percentage in is 30-35%; wherein, the polyethylene glycol is PEG-2000.
[0025] Furthermore, as a preferred solution, in the lactic acid-glycolic acid copolymer, the mass ratio of the two monomers is 75:25-50:50. With this preferred proportion of the polymer as the matrix, a more stable and lasting release effect will be obtained.
[0026] A method for preparing LID-PEG-PLGA slow-release nano-microspheres, the LID-PEG-PLGA slow-release nano-microspheres described in the method are the above-mentioned slow-release nano-microspheres in this specific emb...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com