Method for preparing nano porous copper by adopting Cu-Zn alloy
A technology of nanoporous copper and cu-zn, which is applied in the field of preparing nanoporous copper, can solve the problems of uneven pore size, high price, unclear ligament contour, etc., and achieve the effect of small and uniform pore size and clear ligament contour.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
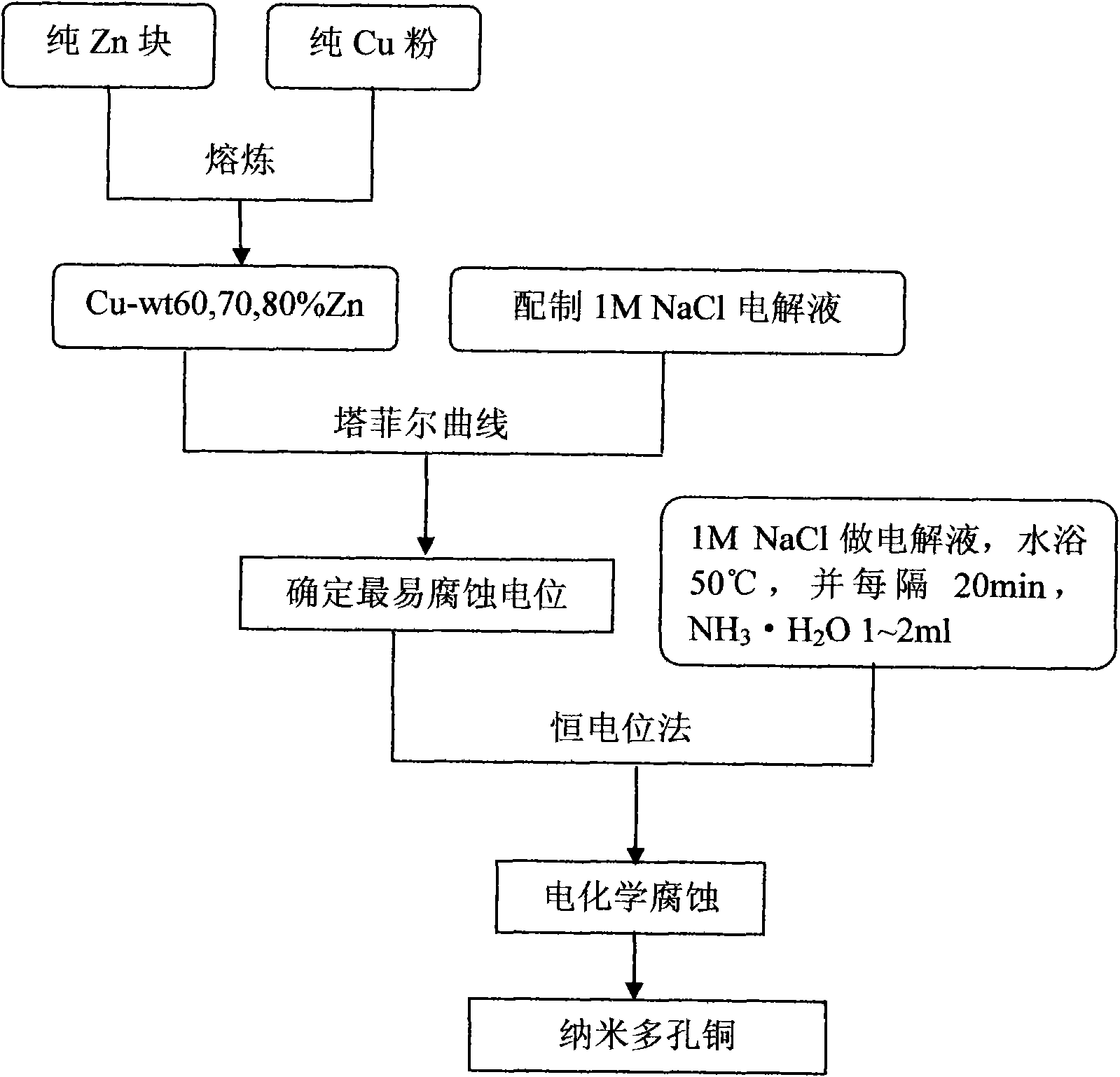
[0019] Weigh copper powder 60g (purity 99.9%) and pure zinc block 90g (purity 99.9%) by weight percentage Cu-wt60%Zn, place in a graphite crucible, and smelt under nitrogen protection. The main process of smelting is: from room temperature, heating to 520°C after 20 minutes, holding at 520°C for 40 minutes, heating to 950°C after 40 minutes, holding at 950°C for 60 minutes, cooling to 350°C after 120 minutes, Keep the temperature at 350°C for 120 minutes, and then cool down to room temperature with the furnace to obtain Cu-wt60%Zn alloy.
[0020] Prepare 1M NaCl solution as the electrolyte, and use the calomel electrode as the reference electrode and the platinum electrode as the auxiliary electrode in the CHI660D electrochemical workstation, and use the three-electrode method to test the Tafel curve to obtain the alloy Cu-wt60%Zn The most susceptible to corrosion voltage is 0.9V.
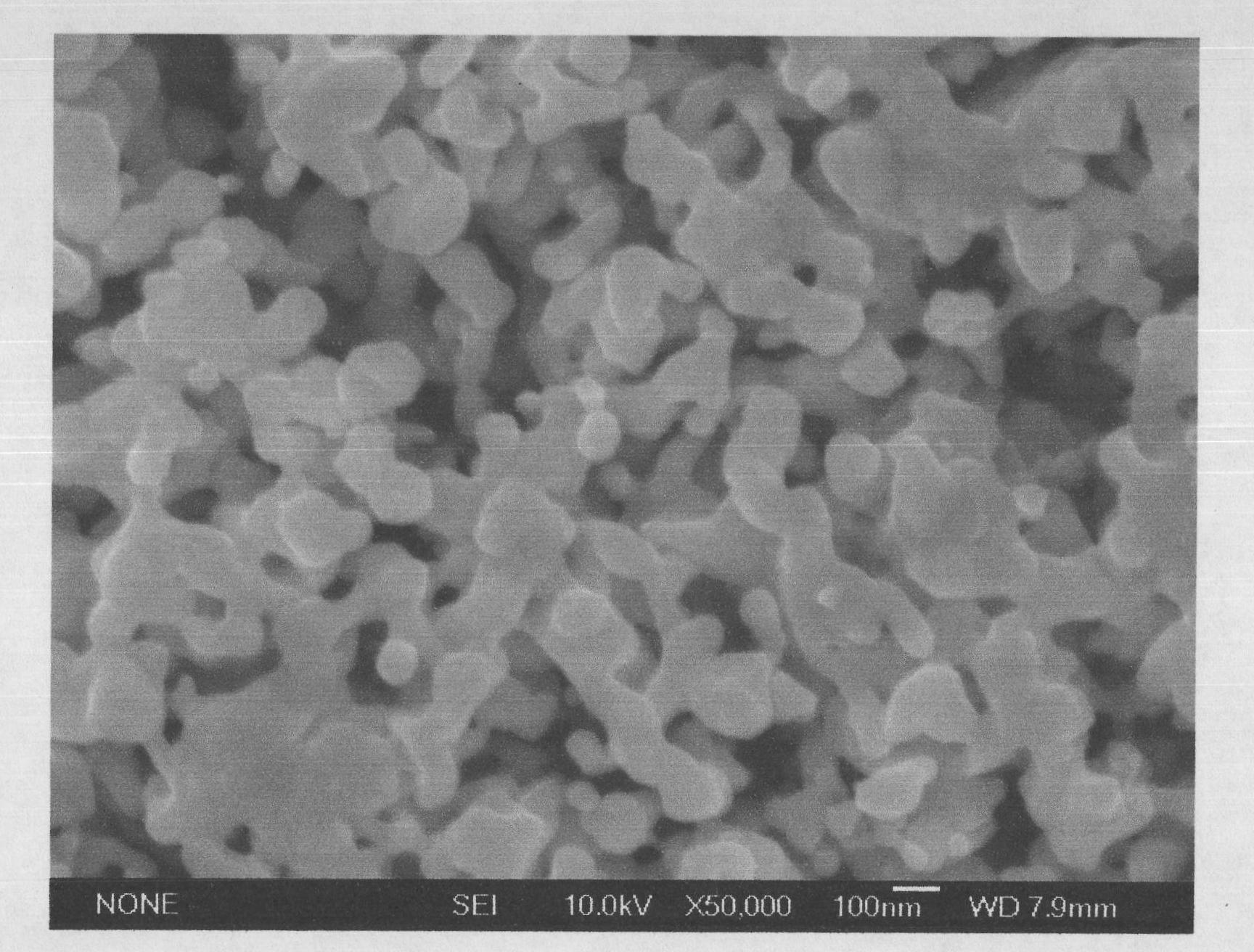
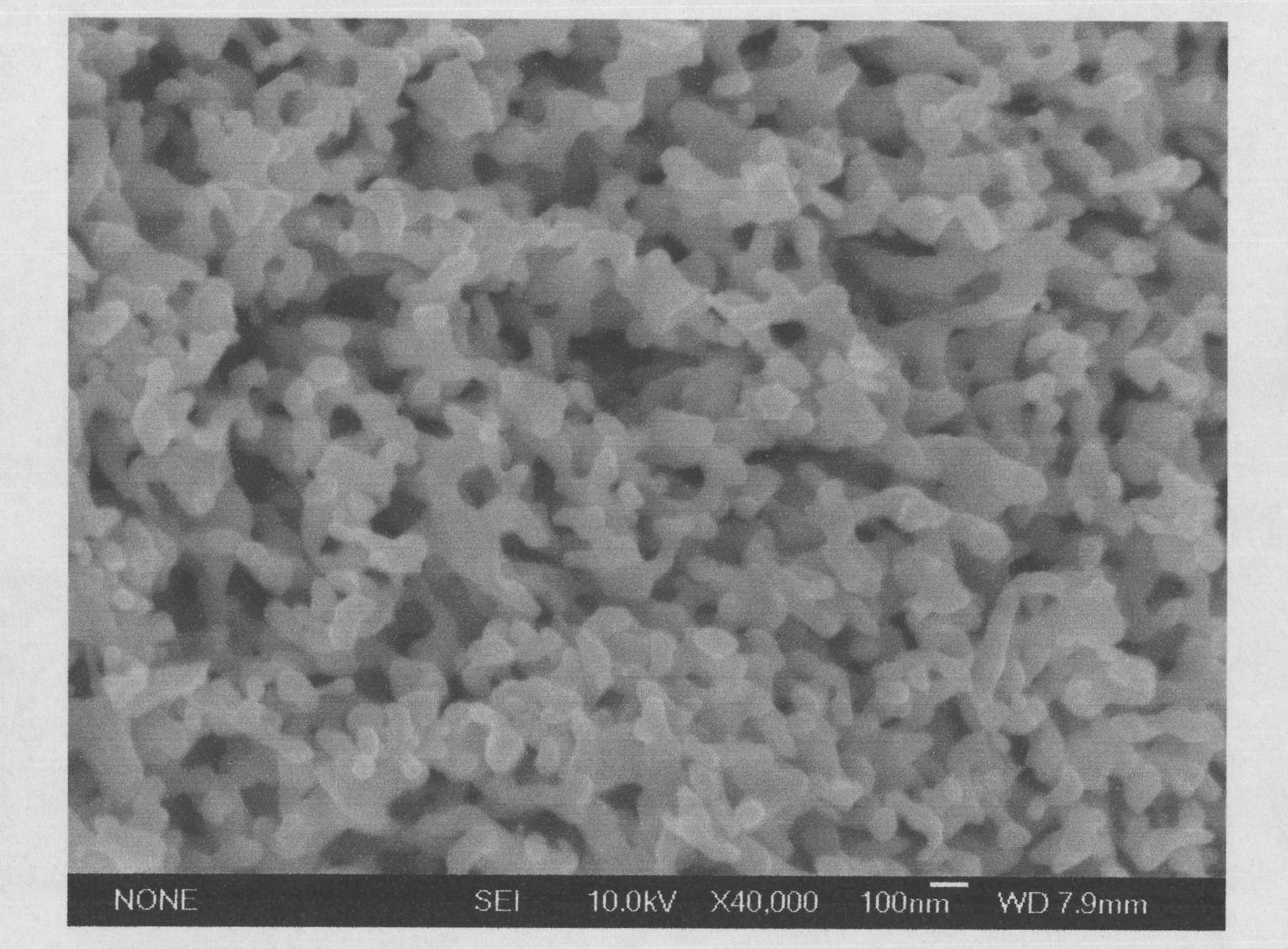
[0021] Using the most easily corroded voltage obtained in the previous step, in the MCP-1 pote...
Embodiment 2
[0023] Weigh copper powder 45g (purity 99.9%) and pure zinc block 105g (purity 99.9%) by weight percentage Cu-wt70%Zn, place in a graphite crucible and melt under nitrogen protection. The main process of smelting is: from room temperature, heating to 510°C after 25 minutes, holding at 510°C for 35 minutes, heating to 940°C after 35 minutes, holding at 940°C for 50 minutes, cooling to 340°C after 110 minutes, Keep the temperature at 340°C for 110 minutes, and then cool down to room temperature with the furnace to obtain Cu-wt70%Zn alloy.
[0024] Prepare 1M NaCl solution as the electrolyte, and use the calomel electrode as the reference electrode and the platinum electrode as the auxiliary electrode in the CHI660D electrochemical workstation, and use the three-electrode method to test the Tafel curve to obtain the alloy Cu-wt70%Zn The most corrosive voltage is 1.0V.
[0025] Using the most easily corroded voltage obtained in the previous step, in the MCP-1 potentiostat, the ca...
Embodiment 3
[0027] Weigh copper powder 30g (purity 99.9%) and pure zinc block 120g (purity 99.9%) by weight percentage Cu-wt80%Zn, place in a graphite crucible and melt under nitrogen protection. The main process of smelting is: from room temperature, heating to 500°C in 30 minutes, holding at 500°C for 30 minutes, heating to 920°C in 30 minutes, holding at 920°C for 60 minutes, cooling to 330°C in 100 minutes, Keep the temperature at 330°C for 100 minutes, and then cool down to room temperature with the furnace to obtain Cu-wt80%Zn alloy.
[0028] Prepare 1M NaCl solution as the electrolyte, and use the calomel electrode as the reference electrode and the platinum electrode as the auxiliary electrode in the CHI660D electrochemical workstation, and use the three-electrode method to test the Tafel curve to obtain the alloy Cu-wt80%Zn The most susceptible to corrosion voltage is 1.25V.
[0029] Using the most easily corroded voltage obtained by the above method, in the MCP-1 potentiostat, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com