Preparation method of potassium ferrate
A technology of potassium ferrate and potassium hypochlorite, applied in chemical instruments and methods, iron compounds, inorganic chemistry and other directions, can solve the problems of long time consumption, low success rate, and difficulty in eliminating potential safety hazards, and achieves reduction of environmental pollution and production cycle. Short, fast-response effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
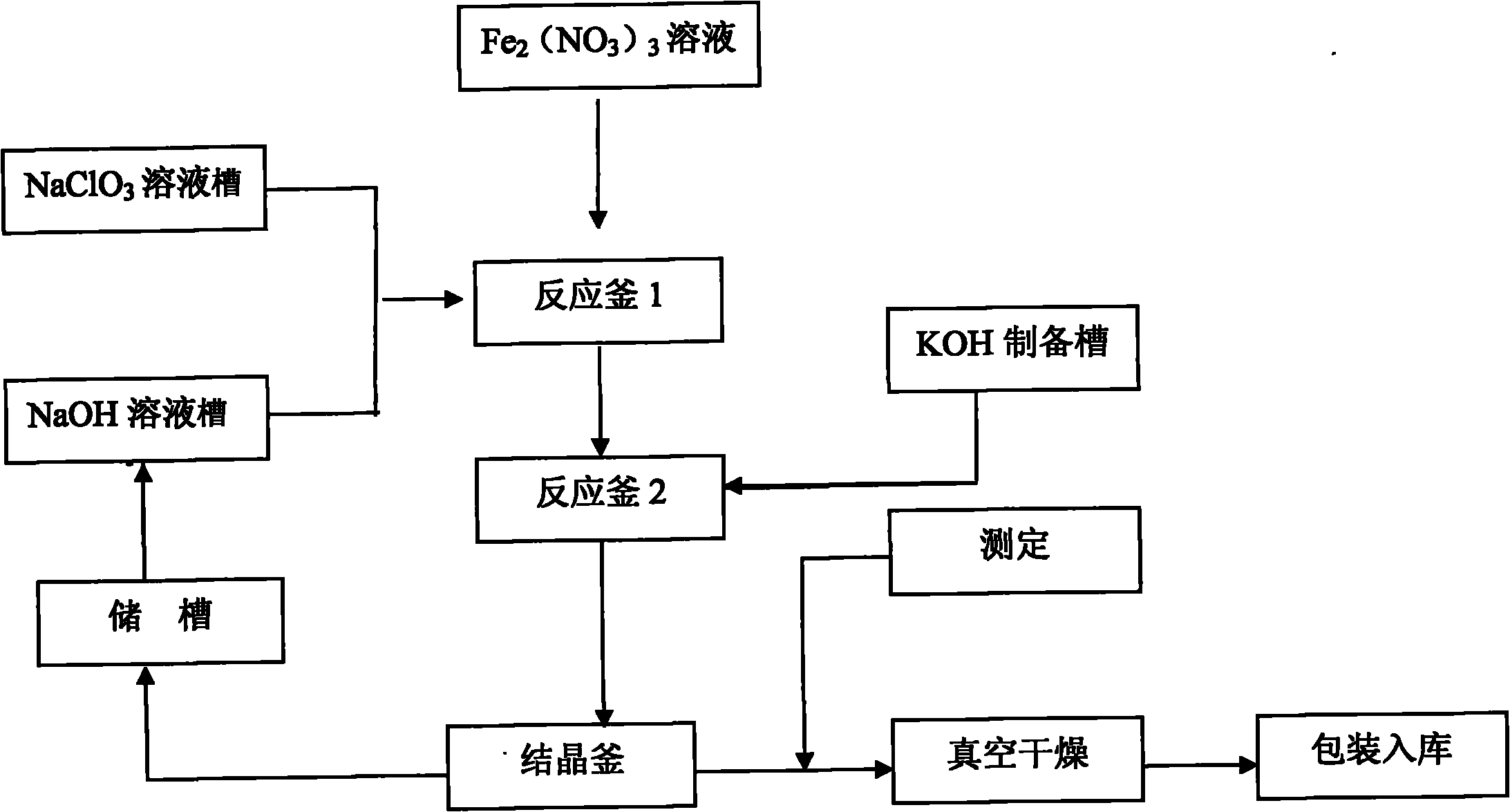
[0023] Such as figure 1 As shown, the method for preparing potassium ferrate of the present invention includes the following steps:
[0024] Step 1. Mix the NaOH and NClO solutions in the reactor, add dropwise iron nitrate Fe(NO 3 ) 3 Solution, the best condition for preparing potassium ferrate is that the mass concentration of NaClO (potassium hypochlorite) is 270g / L, Fe(NO 3 ) 3 The mass concentration is 330g / L;
[0025] Step 2. The optimal oxidation reaction temperature for preparing potassium ferrate is 26°C;
[0026] Step 3. The optimal oxidation reaction time for preparing potassium ferrate is 1.5h;
[0027] The reaction formula is:
[0028] 3NaClO+2Fe(NO 3 ) 3 +10NaOH=2Na 2 FeO 4 +3NaCl+6NaNO 3 +5H 2 O
[0029] Na 2 FeO 4 +2koH=K 2 FeO 4 +2NaOH
[0030] Step 4. The crude potassium ferrate is recrystallized and washed with isopropanol;
[0031] Step 5. The washed crystals are dried under vacuum conditions, the vacuum degree is controlled to 80KPa, and the drying temperature is 70°C;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com