Preparation and use of truncated Cap protein of porcine circovirus type 2
A porcine circovirus and truncated type technology, applied in the field of genetic engineering, can solve the problems of restricted expression, high price, low expression of PCV2Cap protein, etc., and achieve the effect of solving the problem of high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: the preparation method of porcine circovirus type 2 truncated Cap protein vaccine
[0043] 1. Cloning the porcine circovirus type 2 truncated Cap protein gene into an expression vector to obtain a recombinant expression vector.
[0044] ① Design of primers
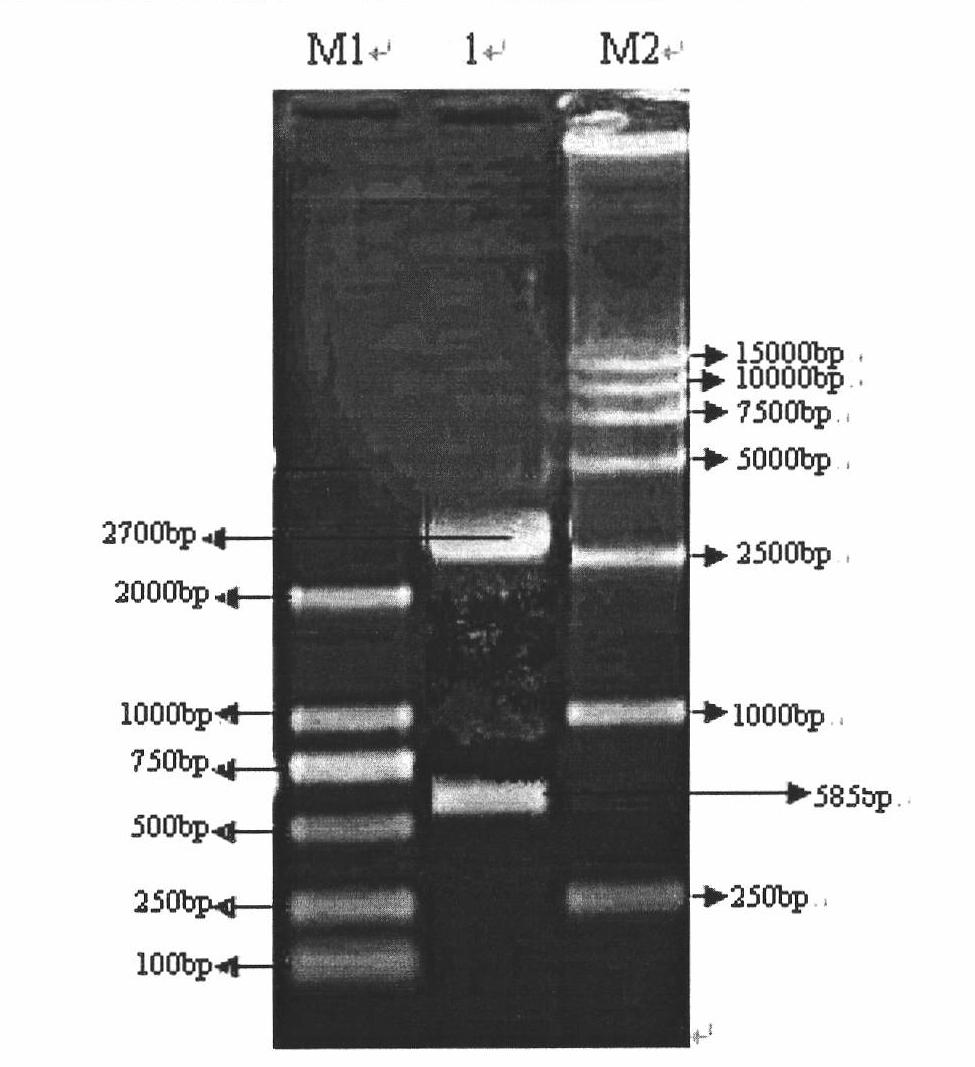
[0045] Using the PCV2-SH strain nucleic acid sequence as a reference (GenBank: AY686763), use Oligo6.0 primer design software to design porcine circovirus type 2 truncated Cap gene-specific primers, upstream primers: restriction site NcoI 5′-CATGCCATGGTGAATGGCATCTTC -3'; downstream primer: restriction site HindIII 5'-CCCAAGCTTTTAAGGGTTAAGT-3', the length of the target gene for PCR amplification is 585bp.
[0046] ② Extraction of PCV2 virus nucleic acid
[0047] 1ml DNAzol Reagent (Invitrogen ) into 0.1ml of the PCV2-SH virus strain sample with the preservation number CGMCCNo.2389, shake the centrifuge tube upside down; let stand at room temperature for 5min; centrifuge at 4°C (10,000r / min, 10min), ...
Embodiment 2
[0068] Example 2: Efficacy and biological testing of porcine circovirus type 2 truncated Cap protein vaccine
[0069] The oil-emulsion vaccine prepared in Example 1 was tested for finished vaccines in accordance with the requirements of the veterinary biological products regulations, mainly for character testing, safety testing, efficacy testing, and determination of formaldehyde and thimerosal residues for the finished vaccines. The results showed that the sampling samples had a uniform appearance and no mold growth; the safety inspection and efficacy inspection all met the requirements; the residues of formaldehyde and thimerosal also met the requirements. Use qualified products to carry out clinical trials - piglet challenge tests.
[0070] ①Safety test
[0071] Five 25-day-old piglets (provided by a pig farm in Jiangsu) were inoculated intramuscularly with 2 times the dose of PCV2Cap vaccine (4ml / head), and there was no abnormal reaction after 28 days of clinical observat...
Embodiment 3
[0074] Example 3 Identification of immunogenicity of porcine circovirus type 2 truncated Cap protein and its application in the preparation of detection reagents for PCV2
[0075] ①Western-blot test
[0076] The purified porcine circovirus type 2 truncated Cap protein was identified by Western-blot. The molecular weight of the truncated PCV2 Cap protein is about 22.5Ku, and it has a positive result with porcine circovirus type 2 positive seroreaction; it has a negative result with negative seroreaction; Positive sera of porcine pseudorabies and porcine parvovirus have no reaction, the results are shown in Figure 5 . Among them, 1-6 were positive for porcine circovirus type 2 truncated Cap protein and swine fever, porcine reproductive and respiratory syndrome, porcine pneumonia, porcine pseudorabies, porcine parvovirus disease positive serum and porcine circovirus type 2 negative Serum reaction result; 7 is positive serological reaction result of porcine circovirus type 2 t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Gene length | aaaaa | aaaaa |
| Expression | aaaaa | aaaaa |
| Expression | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com