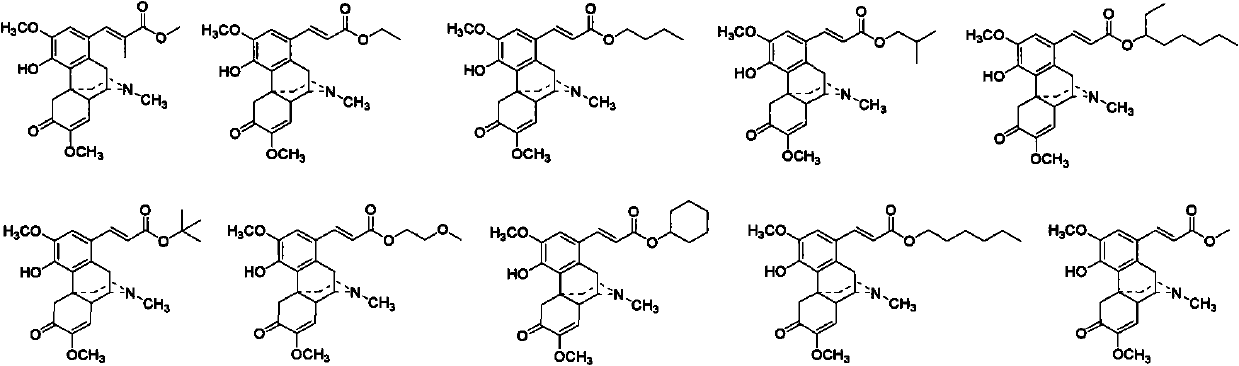
Sinomenine derivative and preparation method and applications thereof
A technology of sinomenine and its derivatives, applied in the field of sinomenine derivatives and its preparation, can solve the problems of no report on the structure modification of sinomenine, and achieve good anti-inflammatory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the synthesis of 1-chlorosinomenine
[0029] Dilute the chemically pure Antiformin solution to 25% (v / v), slowly add 50mL dropwise to 20mL aqueous solution of 2g sinomenine hydrochloride 1 within 10min under stirring, after the dropwise addition is completed , adjust pH=9~10 with hydrochloric acid, extract with dichloromethane (3×20mL), wash with anhydrous Na 2 SO 4 Drying, rotary evaporation, to obtain crude product, column chromatography purification (DCM / CH 3 OH, v / v=15:1) to obtain 1-chlorosinomenine 1, yield: 58%.
[0030]
[0031] mp: 179~181℃; ESI-MSm / z: 364, 366[M+H] + ; 1 HNMR (300MHz; CDCl 3 ; TMS) δ: 6.72 (1H, s), 5.48 (1H, d, J = 1.8Hz), 4.37 (1H, d, J = 15.6Hz), 3.76 (3H, s), 3.49 (3H, s), 3.32(1H, m), 3.09(1H, m), 3.05(1H, m), 2.53(3H, m), 2.42(3H, s), 1.96(3H, m)ppm; 13 CNMR (75MHz; CDCl 3 ; TMS) δ: 193.7, 152.3, 145.3, 143.5, 127.4, 124.0, 122.9, 114.6, 110.0, 56.0, 55.9, 54.8, 48.9, 46.8, 45.4, 42.6, 40.7, 35.5, 22.8ppm.
Embodiment 2
[0032] Embodiment 2: the synthesis of 1-bromosinomenine
[0033]At room temperature, dissolve 2.2g sinomenine in 50mL of dichloromethane, slowly add 1.25g of NBS within 5min under stirring, continue the reaction at 50°C for 30min, after cooling, add 40mL of Na 2 S 2 o 3 The saturated solution was stirred for 5 min, and the dichloromethane layer was separated, washed with an appropriate amount of water and saturated brine, and washed with anhydrous Na 2 SO 4 After drying and rotary evaporation, 1-bromosinomenine 2 was obtained as a pale yellow solid with a yield of 93%.
[0034]
[0035] mp: 134-136°C; ESI-MS m / z: 407, 408 [M-H] - ; 1 HNMR (300MHz; CDCl 3 ; TMS) δ: 6.92 (1H, s), 5.44 (1H, d, J = 1.8Hz), 4.34 (1H, d, J = 15.6Hz), 3.81 (3H, s), 3.50 (3H, s), 3.33(1H, m), 3.12(1H, m), 3.01(1H, d, J=18.9Hz), 2.62(1H, m), 2.48(5H, m), 1.97(3H, m)ppm; 13 CNMR (75MHz; CDCl 3 ; TMS) δ: 193.8, 152.8, 146.0, 144.5, 128.9, 124.6, 114.5, 113.6, 113.4, 57.0, 56.5, 55.2, 49.1, 47...
Embodiment 3
[0036] Embodiment 3: the synthesis of 1-iodosinomenine
[0037] In 80mL of dichloromethane, add 3.29g (10mmol) of sinomenine, after dissolving, add 2.25g (10mmol) of N-iodosuccinimide, stir at room temperature for 2 hours, add an appropriate amount of saturated sodium thiosulfate solution Wash the dichloromethane solution, and then wash the dichloromethane solution with saturated brine (3×20mL), dry over anhydrous sodium sulfate, concentrate by rotary evaporation, and separate on a silica gel column. The developing solvent is: dichloromethane / methanol to obtain 1- Iodosinomenine 3 (4.14 g, 92% yield).
[0038]
[0039] Yield: 92%; mp: 106-108°C; ESI-MSm / z: 454[M-H]-; 1HNMR (300MHz; CDCl3; TMS) δ: 7.16 (1H, s), 6.58 (1H, br), 5.44 (1H, s), 4.35(1H, d, J=15.6Hz), 3.80(3H, s), 3.50(3H, s), 3.37(1H, m), 3.14(1H, m), 2.90(1H, d, J=18.9Hz), 2.66 (1H, m), 2.49 (5H, m), 2.00 (3H, m) ppm; 13CNMR (75MHz; CDCl3; TMS) δ: 194.0, 152.6, 146.1, 145.5, 132.3, 124.7, 119.6, 115.0, 88.1, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com