Parnaparin production method
A production method and parparparin technology are applied in the field of preparation of biochemical drugs to achieve the effects of high yield, low bleeding risk and good product stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
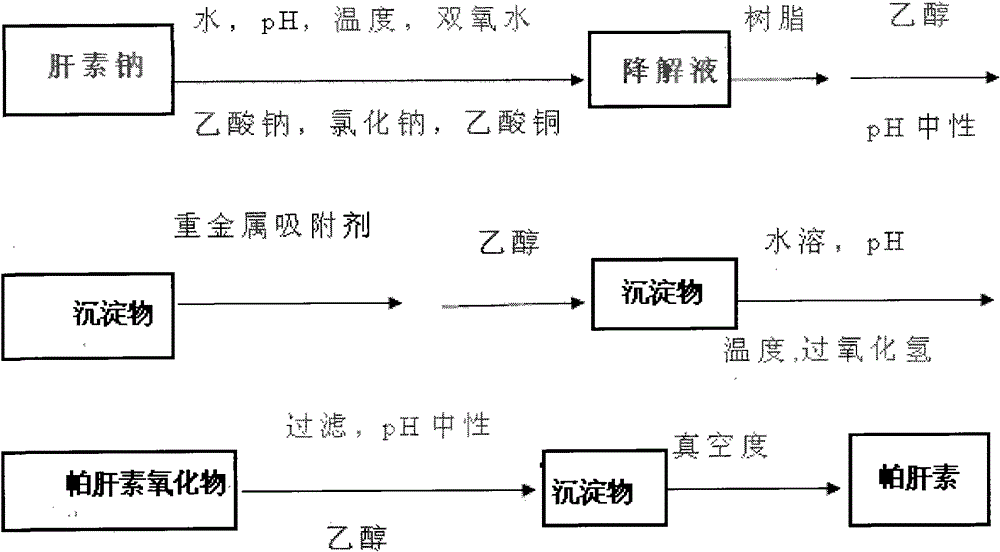
[0007] combined with figure 1 , to further describe the present invention:
[0008] Take heparin sodium and dissolve in purified water to a concentration of 2% to 3%, add sodium acetate and sodium chloride, adjust the pH to 7 to 8, add dissolved copper acetate, and add 2% to 3% hydrogen peroxide (v / v volume ratio, the same below), put it in a water bath at 50-55°C, and start the reaction, during which the pH and temperature are controlled. After 18-24 hours of degradation reaction, adjust the pH to 9.5-10.0, add EDTA.2Na (disodium ethylenediamine tetraacetate), and react for 4 hours, adjust the pH to neutral, precipitate with 3 times the amount of ethanol, and place it for 21 hours. Dissolve the resulting precipitate to a concentration of 10% to 15%, and treat it with a strong acidic cationic resin, such as a 001X7 resin bed, collect the effluent, and adjust the pH to neutral. After the resin treatment is completed, add 3 times the amount of medicinal Ethanol precipitation, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com