Organism coupling preparation method of R(-)-4-cyan-3-hydroxybutyric acid ethyl ester
A technology of ethyl hydroxybutyrate and hydroxybutyronitrile is applied in the field of biological coupling preparation of fine chemicals, which can solve the problems of limited industrial application, complicated process and need for recrystallization treatment, and achieve obvious economic and environmental benefits. High purity and optical purity, realizing the effect of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
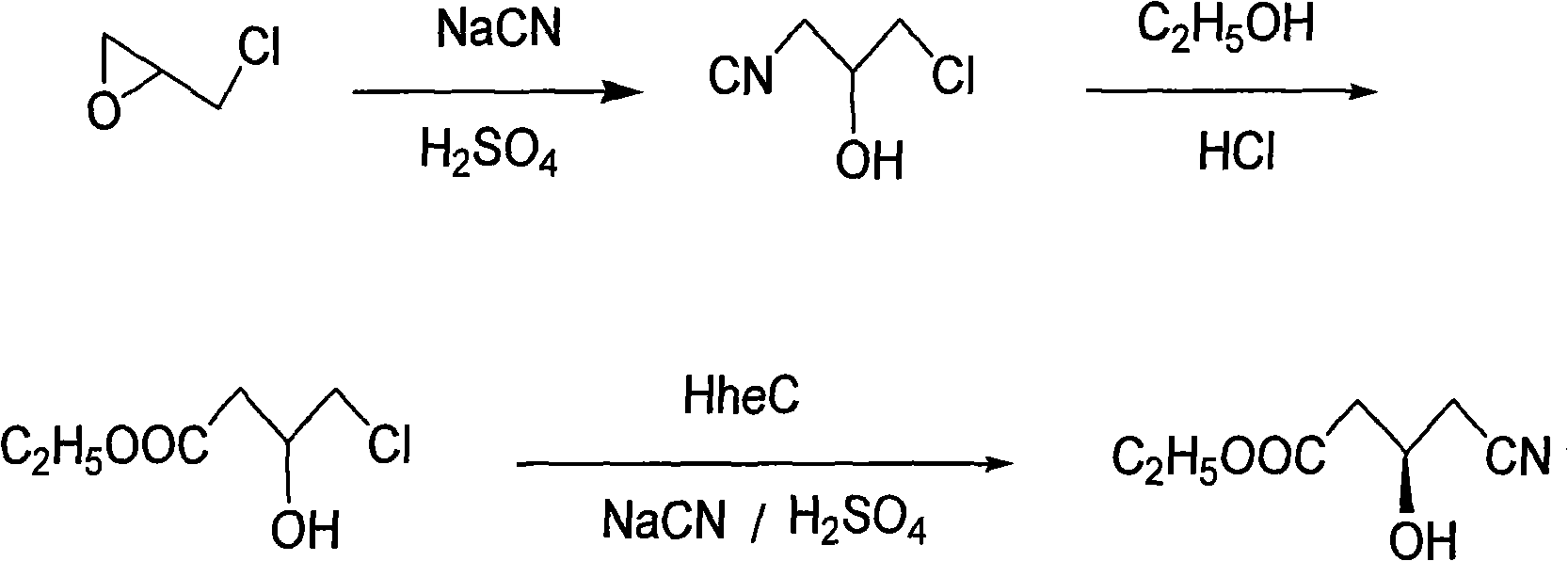
[0033] Example 1. The biocoupling preparation method of R(-)-4-cyano-3-hydroxybutyrate ethyl ester, its steps are as follows:
[0034] (1) Preparation of 4-chloro-3-hydroxybutyronitrile: Add epichlorohydrin to the buffer solution made of buffer dissolved in water, control the pH value of the reaction solution to 6, control the reaction temperature to 10°C, and drop Sulfuric acid solution and sodium cyanide solution, insulation reaction 2 hours after dropwise addition, organic solvent dichloromethane extracts and desolventizes to obtain product; The molar ratio of substance is: epichlorohydrin: sulfuric acid: sodium cyanide: buffering agent=1: 1.0:1.0:0.1; the buffer used is disodium hydrogen phosphate-sodium dihydrogen phosphate (1:1);
[0035] (2) Preparation of ethyl 4-chloro-3-hydroxybutyrate: add ethanol hydrochloride to 4-chloro-3-hydroxybutyronitrile, react at 20°C for 4 hours, then precipitate and filter, and the resulting filtrate is the product; The weight content o...
Embodiment 2
[0037] Example 2. The biocoupling preparation method of R(-)-4-cyano-3-hydroxybutyrate ethyl ester, its steps are as follows:
[0038] (1) Preparation of 4-chloro-3-hydroxybutyronitrile: Add epichlorohydrin to the buffer solution made by dissolving the buffer in water, control the pH value of the reaction solution to 10, control the reaction temperature to 50°C, and add dropwise Sulfuric acid solution and sodium cyanide solution, after dropwise addition, keep warm for 6 hours, and extract the product after extraction with organic solvent toluene; the molar ratio of substances is: epichlorohydrin: sulfuric acid: sodium cyanide: buffer = 1: 1.6: 1.6:0.5; the buffer used is boric acid-potassium chloride (1:1);
[0039] (2) Preparation of ethyl 4-chloro-3-hydroxybutyrate: add ethanol hydrochloride to 4-chloro-3-hydroxybutyronitrile, react at 80°C for 10 hours, then precipitate and filter, and the resulting filtrate is the product; The weight content of hydrochloric acid in ethan...
Embodiment 3
[0041] Example 3. The biocoupling preparation method of R(-)-4-cyano-3-hydroxybutyrate ethyl ester, its steps are as follows:
[0042] (1) Preparation of 4-chloro-3-hydroxybutyronitrile: Add epichlorohydrin to the buffer solution made of buffer dissolved in water, control the pH value of the reaction solution to 8.5, control the reaction temperature to 25°C, and drop Sulfuric acid solution and sodium cyanide solution, insulation reaction 5 hours after dropping, organic solvent ethyl acetate extracts and desolventizes to obtain product; The molar ratio of substance is: epichlorohydrin: sulfuric acid: sodium cyanide: buffering agent=1: 1.3: 1.3: 0.4; the buffer used is tris;
[0043] (2) Preparation of ethyl 4-chloro-3-hydroxybutyrate: add ethanol hydrochloride to 4-chloro-3-hydroxybutyronitrile, react at 60°C for 6 hours, then precipitate and filter, and the resulting filtrate is the product; The weight content of hydrochloric acid in ethanol hydrochloride is 30%; The weight ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com