Method for preparing S-benzyl cysteinyl glycine ethyl ester under microwave condition
A technology of cysteinyl glycine ethyl ester and glycine ethyl ester hydrochloride, which is applied in the preparation of sulfides, organic chemistry, etc., can solve the problems of easy acid hydrolysis, difficult preservation of raw materials, and inability to obtain target products, etc. High efficiency, short reaction time and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
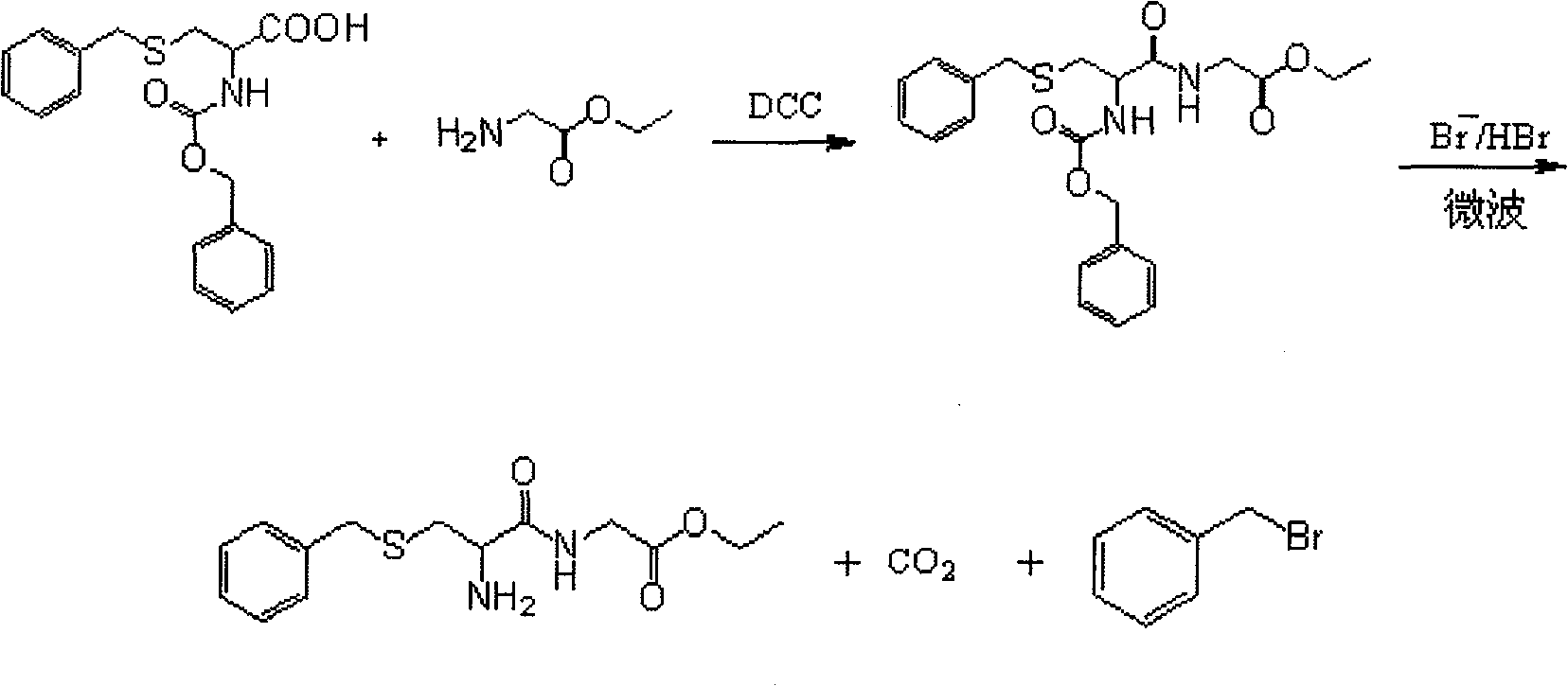
[0025] 1) Synthesis of S-benzyl-N-benzyloxycarbonylcysteinyl glycine ethyl ester
[0026] Glycine ethyl ester hydrochloride (2) 6.98g (0.05mol), was dissolved in 200ml of dichloromethane, and 20ml of tetrahydrofuran and 7ml of triethylamine were added to the solution, stirred to form a homogeneous solution, then 17.3g (0.05mol) was added ) S-benzyl-N-benzyloxycarbonyl cysteine to form a uniform and transparent solution, control its temperature at about -2°C, and take another 10.5ml (0.05mol) of DCC and dissolve it in 50ml of dichloromethane, and stir Under certain conditions, slowly add it dropwise to the above-mentioned mixed solution of A and B, and control the dropping speed. After about 1 hour of dropping, keep it at -2°C for 5 hours. Remove N,N`-dicyclohexyl urea (DCU) by filtration, and the filtrate is washed with dilute HCl, NaHCO 3 Wash each time with anhydrous NaSO 4 Drying, concentration under reduced pressure, and solidification of petroleum ether gave 16.38 g o...
Embodiment 2
[0030] 1) same as embodiment 1;
[0031] 2) Synthesis of S-benzylcysteinyl glycine ethyl ester
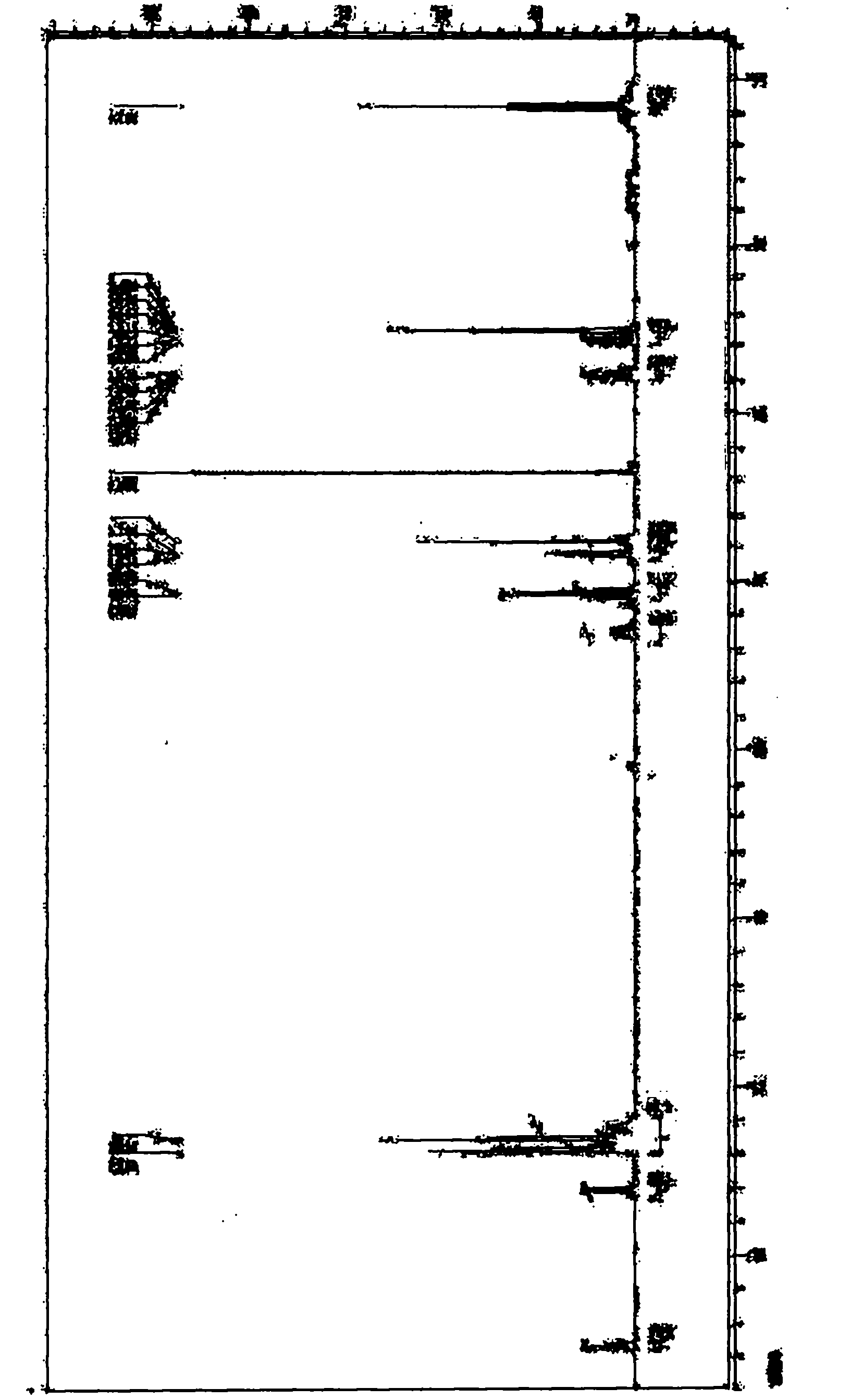
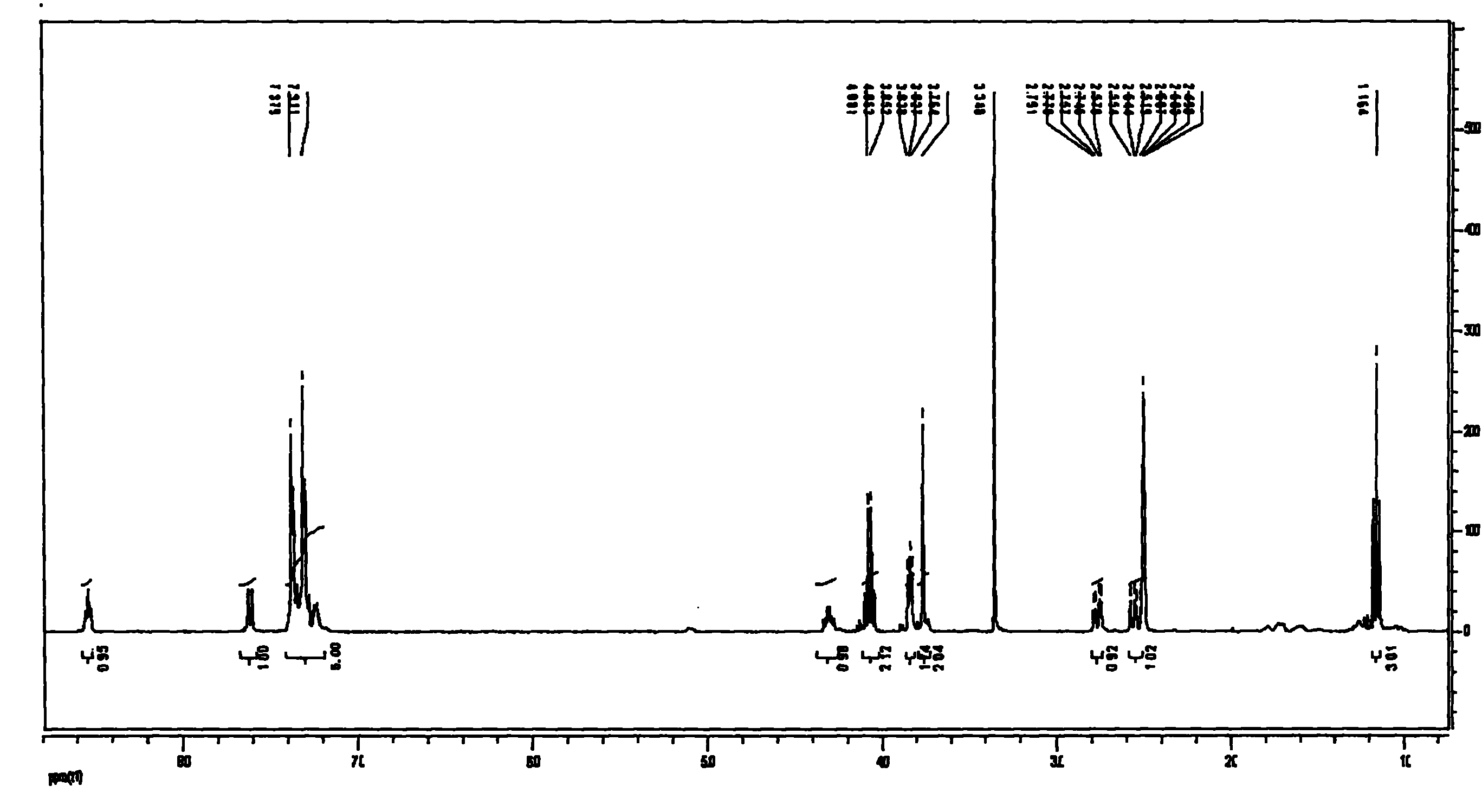
[0032] Add 5g of S-benzyl-N-benzyloxycarbonylcysteinylglycine ethyl ester (3) in a 100ml three-necked flask equipped with a reflux condenser (a balloon is connected above the condenser to prevent hydrogen bromide from volatilizing too quickly), In a microwave instrument (built-in magnetic stirring), then add 25ml of 33% hydrogen bromide in acetic acid solution at 30°C, the reactant dissolves quickly and releases gas, the volume of the balloon expands, and after 15 minutes, part of the hydrogen bromide is pumped out under reduced pressure and glacial acetic acid to obtain an orange transparent liquid, add ether to wash to remove residual benzyl bromide, add water to the water layer and filter to remove insoluble matter, and freeze-dry the filtrate to obtain the product S-benzylcysteinylglycine ethyl ester (4): 2.83 g of slightly orange crystals, yield 82.3%.
Embodiment 3
[0034] 1) same as embodiment 1;
[0035] 2) Synthesis of S-benzylcysteinyl glycine ethyl ester
[0036] Add 5g of S-benzyl-N-benzyloxycarbonylcysteinylglycine ethyl ester (3) in a 100ml three-necked flask equipped with a reflux condenser (a balloon is connected above the condenser to prevent hydrogen bromide from volatilizing too quickly), In a microwave instrument (built-in magnetic stirring), then add 50ml of 33% hydrogen bromide in acetic acid solution at 30°C, the reactant dissolves quickly and releases gas, the volume of the balloon expands, and after 20 minutes, part of the hydrogen bromide is pumped out under reduced pressure and glacial acetic acid to obtain an orange transparent liquid, add ether to wash to remove residual benzyl bromide, add water to the water layer and filter to remove insoluble matter, and freeze-dry the filtrate to obtain the product S-benzylcysteinylglycine ethyl ester (4): 2.52 g of slightly orange crystals, yield 74.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com