Carveol ester derivative and percutaneous absorption preparation containing same
A technology of carveol and derivatives, applied in the field of medicine, can solve problems that have not been seen yet, achieve low irritation, wide application prospects, and enhance the effect of permeability
Inactive Publication Date: 2010-11-03
SHENYANG PHARMA UNIVERSITY
View PDF2 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
So far, there has not been any research report on the synthesis of new accelerators using carveol as a parent at home and abroad.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
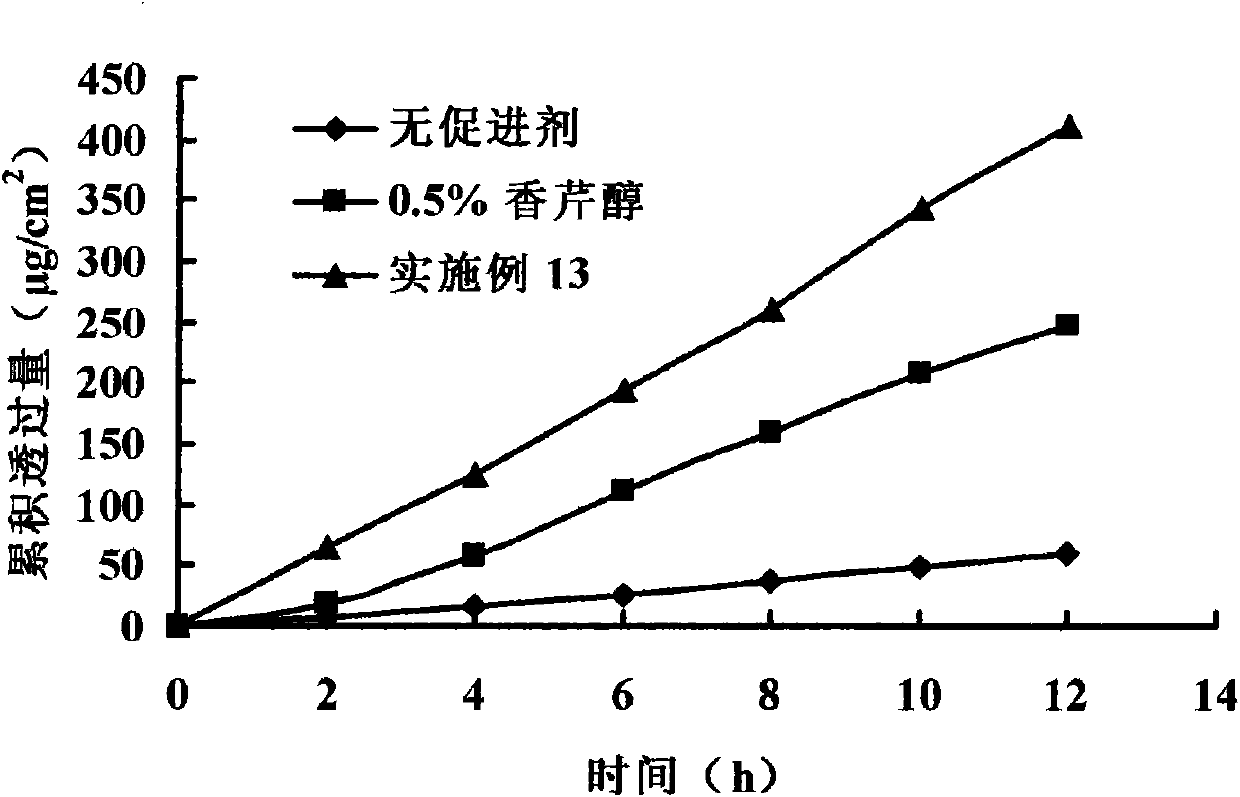
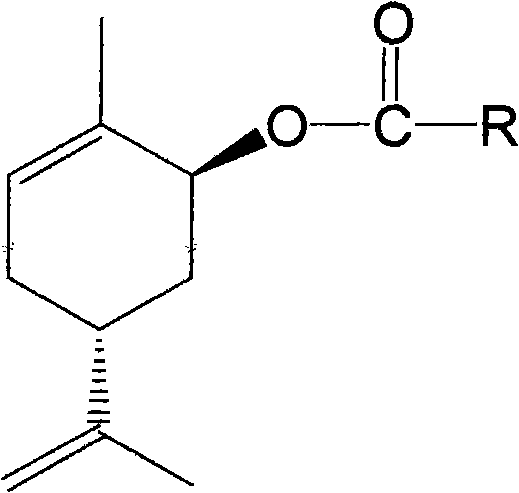
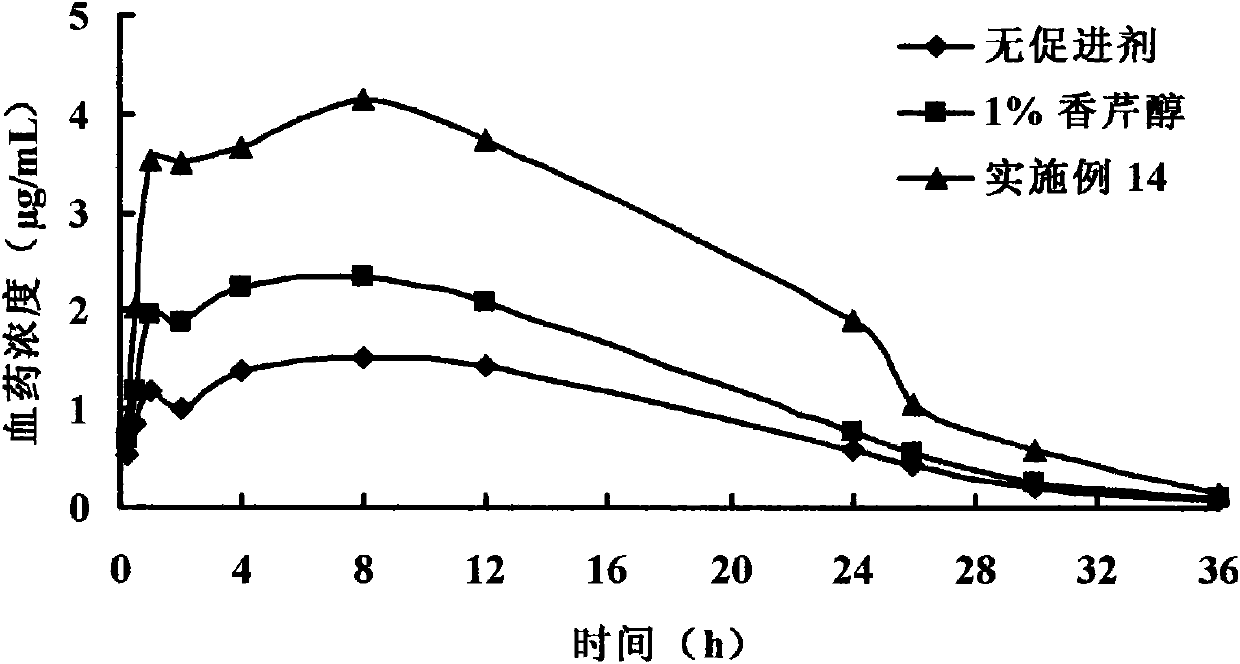
The invention belongs to the technical field of medicines, discloses a carveol ester derivative and a percutaneous administration preparation containing the derivative, and the structure formula thereof is shown in the specification. The derivative comprises saturated and unsaturated fatty acid carveol ester, wherein fatty acid reacts with carveol ester under the catalytic action of p-toluenesulfonic acid to directly form ester, and anhydrous cyclohexane is taken as a solvent. The carveol ester derivative can be taken as a transdermal enhancer for external preparations such as patch, poultice, ointment, gel and the like, thus improving the percutaneous absorptive amount of medicines, and having higher safety, lower irritation and better medicinal penetration capacity.
Description
technical field The invention belongs to the technical field of medicine, relates to a class of carverol ester derivatives and transdermal absorption preparations containing the derivatives, in particular to 8 carverol ester derivatives, including saturated and unsaturated fatty acid carverol esters : heptanoic acid, caprylic acid, capric acid, lauric acid, myristic acid, palmitic acid, stearic acid carveyl ester and oleic acid carveyl ester, its preparation method and in transdermal preparation (patch, Babu formulations, ointments, gels, creams, etc.). Background technique Most of the terpenoid accelerators are derived from natural products (volatile oils). They have the advantages of strong penetration-promoting activity, low toxicity, low irritation, and penetration-promoting effects on both hydrophilic and lipophilic drugs. They have become domestic and foreign percutaneous absorption accelerators. One of the hotspots of research. However, the volatility of terpenoids ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07C69/58C07C69/24C07C67/08A61K31/196A61K31/132A61K31/192A61K31/405A61K9/70A61K9/06A61K9/00A61K9/12A61K47/14
Inventor 方亮王曼丽许永男
Owner SHENYANG PHARMA UNIVERSITY
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Patsnap Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com