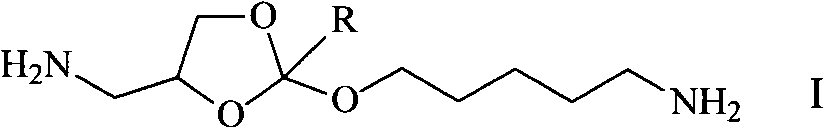
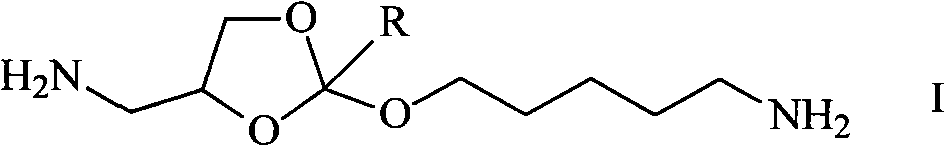
Synthesis method of diamido ortho-ester monomer
A technology of diamino ortho ester and synthesis method, which is applied in the field of synthesis of diamino ortho ester monomer, can solve problems such as long reaction time, uncontrollable molecular weight, sensitivity to light and moisture, and achieve economical and high yield , raw materials are easy to obtain, and the synthesis process is simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The synthetic method of 4-aminomethyl-2-aminopentyloxy-[1,3]dioxane monomer is realized through the following steps:
[0029] 1) Preparation of 2,2,2-trifluoro-N-(2,3-dihydroxypropanol)
[0030] Under a nitrogen atmosphere, add 18.80 g (0.21 mol) of 3-amino-1,2-propanediol and 120 ml of acetonitrile to a 250 ml three-necked flask, and slowly add 34.30 g (0.24 mol) of ethyl trifluoroacetate dropwise at 0°C, at room temperature After reacting for 4 hours and evaporating the volatile reagents under reduced pressure, the crude product was dissolved in ethyl acetate, washed with aqueous potassium bisulfate solution (0.5M) and saturated brine, dried over magnesium sulfate, evaporated under reduced pressure to remove the solvent, and dried in vacuo to obtain colorless The oily pure product was 34.90g, and the yield was 91%. 1 H NMR (300MHz, CD 3 OD): δ (ppm) 3.27-3.29 (m, 2H, NH-CH 2 ), 3.47-3.49 (m, 2H, CH 2 -OH), 3.70-3.78 (m, 1H, CH-OH), 7.60 (b, 1H, NH).
[0031] 2) P...
Embodiment 2
[0038] The synthetic method of 4-aminomethyl-2-aminopentyloxy-2-methyl-[1,3]dioxane monomer is realized through the following steps:
[0039] 1) Preparation of 2,2,2-trifluoro-N-(2,3-dihydroxypropanol)
[0040] Under a nitrogen atmosphere, add 18.80 g (0.21 mol) of 3-amino-1,2-propanediol and 120 ml of acetonitrile to a 250 ml three-necked flask, and slowly add 34.30 g (0.24 mol) of ethyl trifluoroacetate dropwise at 0°C, at room temperature After reacting for 4 hours and evaporating the volatile reagents under reduced pressure, the crude product was dissolved in ethyl acetate, washed with aqueous potassium bisulfate solution (0.5M) and saturated brine, dried over magnesium sulfate, evaporated under reduced pressure to remove the solvent, and dried in vacuo to obtain colorless The oily pure product was 34.90g, and the yield was 91%. 1 H NMR (300MHz, CD 3 OD): δ (ppm) 3.27-3.29 (m, 2H, NH-CH 2 ), 3.47-3.49 (m, 2H, CH 2 -OH), 3.70-3.78 (m, 1H, CH-OH), 7.60 (b, 1H, NH).
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com