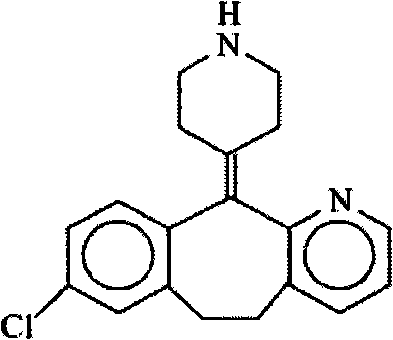
Preparation method of solid pharmaceutical composition containing desloratadine
A technology of desloratadine and its composition, which is applied in the field of preparation of solid pharmaceutical compositions, can solve the problems of insufficient product stability, cumbersome process, and increased quantity, and achieve low density, uniform particle size distribution, and low strength Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
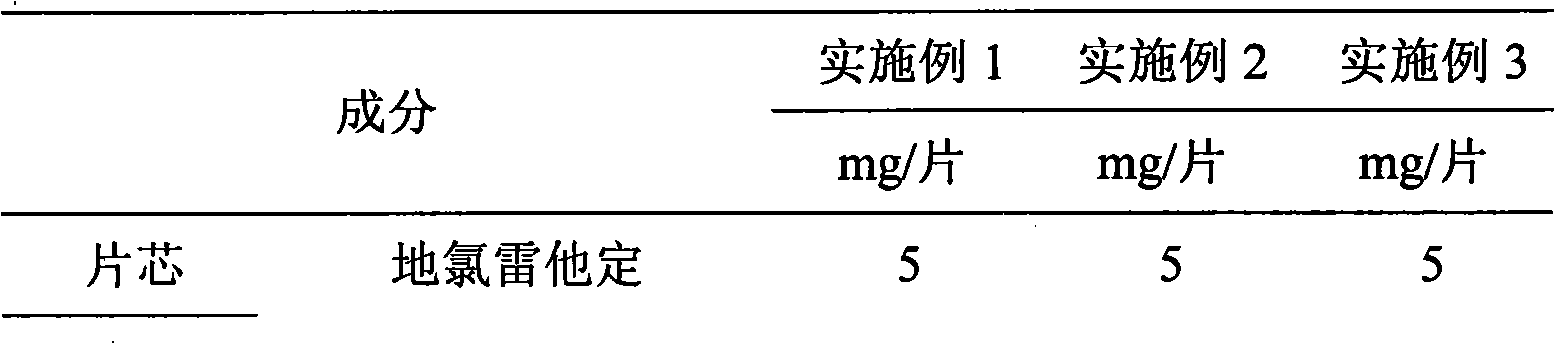
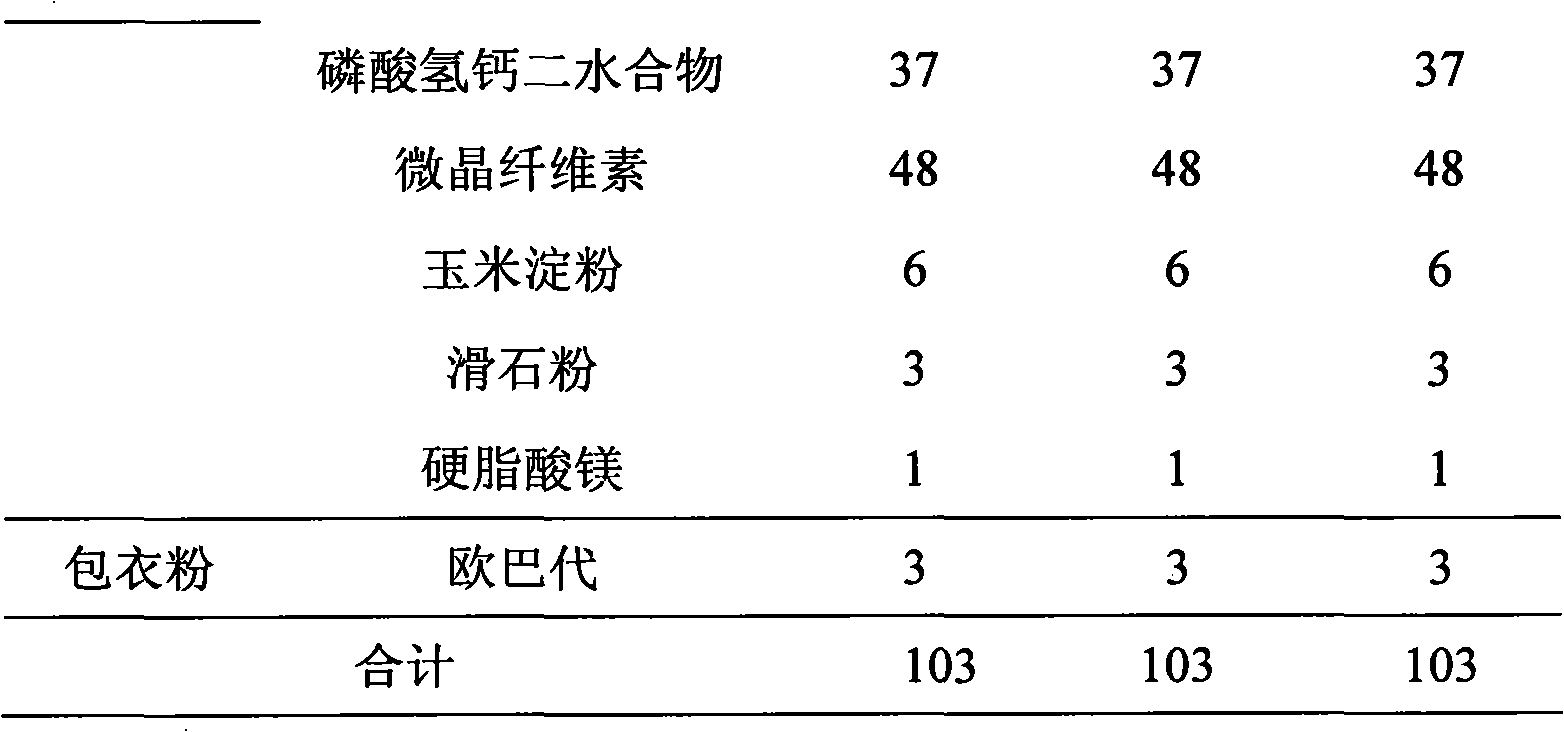
[0070] See Table 3 for the prescription:
[0071] table 3
[0072]
[0073]
[0074] Wherein, the desloratadine particle size d (0.9) that embodiment 1 uses is 150 μ m; The desloratadine particle size d (0.9) that embodiment 2 uses is 330 μ m; The desloratadine that embodiment 3 uses The particle size d(0.9) was 350 μm.
[0075] Preparation Process:
[0076] 1. Disperse 5g of desloratadine into 100g of water to form a dispersion;
[0077] 2. Pour calcium hydrogen phosphate dihydrate, microcrystalline cellulose, and cornstarch into the fluidized bed, and spray the desloratadine aqueous dispersion in a heated fluidized state;
[0078] 3. Heat and dry the material in the fluidized bed so that the drying weight loss of the material is less than 3.0%;
[0079] 4. Sieve the dried material, add talcum powder and magnesium stearate to form a mixture;
[0080] 5. Compress the mixture into tablets with a 7 mm round punch to obtain tablets with a table...
Embodiment 4-6
[0087] See Table 5 for the prescription:
[0088] table 5
[0089]
[0090] Preparation Process:
[0091] 1. Disperse 5g of desloratadine into 100g of water to form a dispersion;
[0092] 2. Pour calcium hydrogen phosphate dihydrate, optimized microcrystalline cellulose, and cornstarch into the fluidized bed, and spray desloratadine water dispersion in a heated fluidized state;
[0093] 3. Heat and dry the material in the fluidized bed so that the drying weight loss of the material is less than 3.0%;
[0094] 4. Sieve the dried material, add talcum powder and magnesium stearate to form a mixture;
[0095] 5. Compress the mixture into tablets with a 7 mm round punch to obtain tablets with a tablet weight of about 100 mg.
[0096] 6. Use a high-efficiency coating pan to coat the tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com