Malaria recombinant antigen, IgY immune body and malaria detection kit
A technology of recombinant antigens and antibodies, applied in measurement devices, resistance to vector-borne diseases, biochemical equipment and methods, etc., can solve the problems of high cost, short half-life, low sensitivity, etc., and achieve significant social and economic benefits. The effect of reducing application cost and avoiding cross-reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
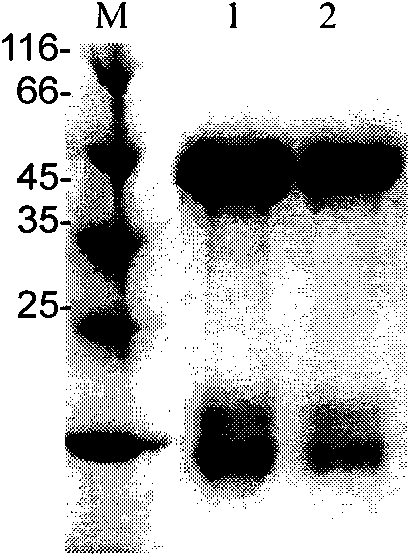
[0032] Example 1: Preparation of Plasmodium falciparum recombinant antigen
[0033]From the analysis of the confirmed specific epitopes of falciparum malaria combined with the malaria genome database, 9 epitopes including five major antigens from RESA, MSA1, CSP, MSA1, and MAg-1 were finally selected, and their sequences are respectively SEQ ID Nos: 1-9, the epitope connection sequence of the antigen designed in the present invention is 9-6-9-8-6-7-7-3-9-6-4-9-1-5-6-2 (numbers are corresponding serial number). Design the polynucleotide sequence encoding the corresponding epitope according to the codon preference of Escherichia coli, according to the primer overlap method known to those skilled in the art, introduce BamH I and Bcl II endonuclease sites respectively at the front and rear ends of the epitope coding sequence, After PCR amplification, the amplified sequence is concatenated in order to form the coding sequence of the recombinant antigen (plus the start codon ATG, i...
Embodiment 2
[0034] Embodiment 2: Preparation of anti-falciparum malaria (Plasmodium falciparum) recombinant antigen IgY
[0035] (1) Antigen immunization Laihang chicken
[0036] 200 μl of the antigen (0.5 mg / ml) obtained in Example 1 was mixed well with an equal volume of Freund's adjuvant to immunize Leghorn chickens (purchased from the School of Veterinary Medicine, China Agricultural University). At week 0, chickens were immunized with complete Freund's adjuvant for primary immunization, and the chicken neck was injected subcutaneously at multiple points; at weeks 3, 5, and 11, chickens were immunized with incomplete adjuvant at multiple points in the leg and wing muscles. Eggs were collected daily and stored at 4°C after labeling for later use.
[0037] (2) Rough purification of IgY
[0038] Wash the fresh Laihang eggs obtained in step (1) and immerse them in 0.1% bromogeramine solution for disinfection, then wipe the shells of the eggs with 75% alcohol cotton. After removing the ...
Embodiment 3
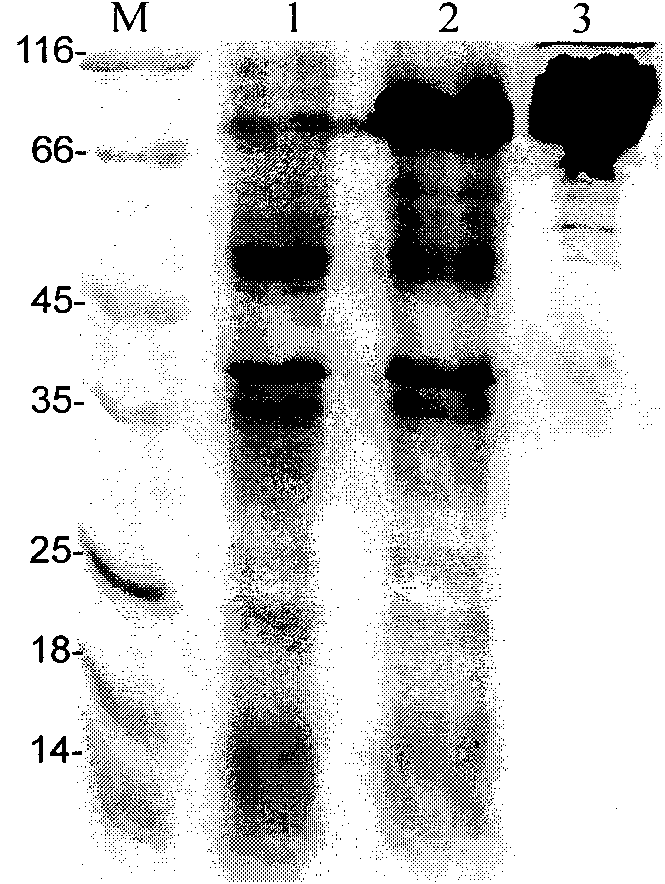
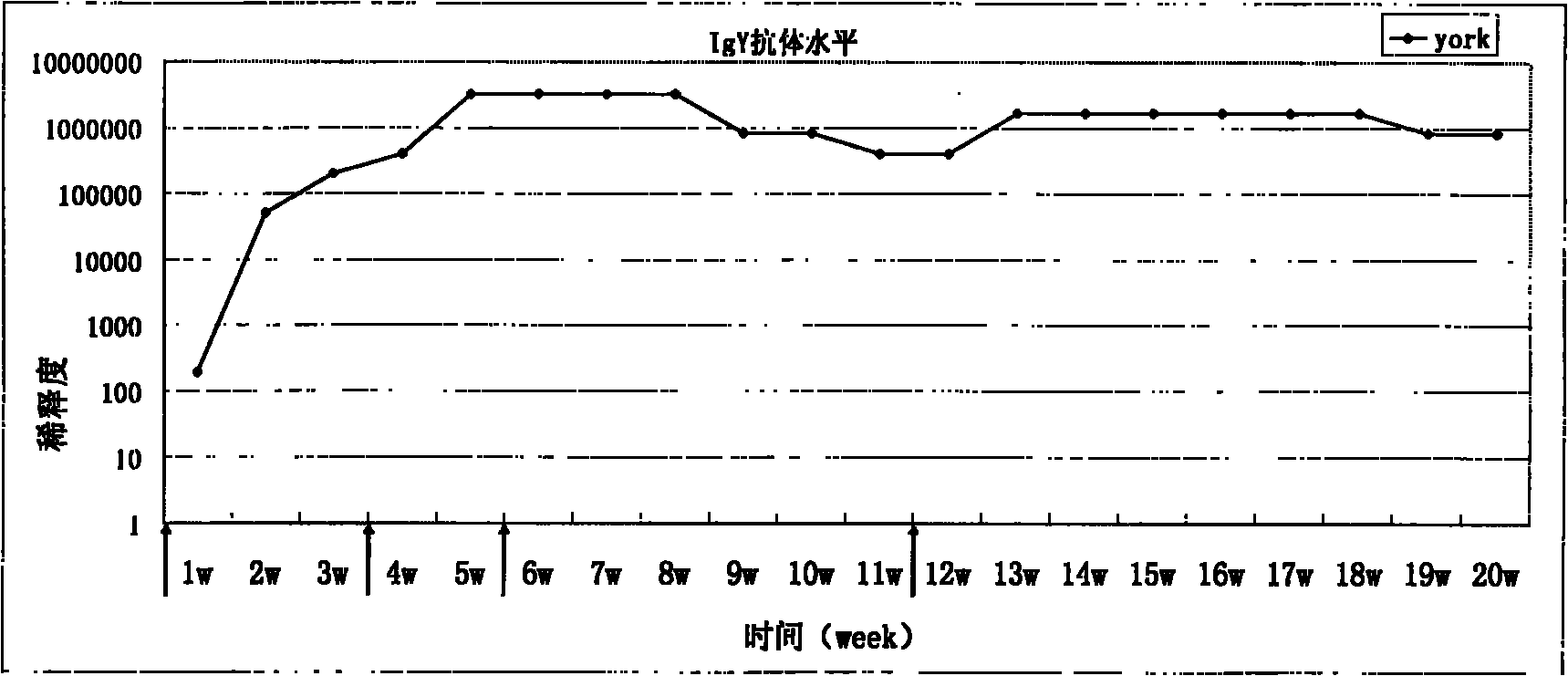
[0044] Example 3: Activity Identification of IgY Antibody
[0045] (1) Determination of the titer of IgY antibody in immunized Laihang chicken eggs:
[0046] Indirect enzyme-linked immunoassay (ELISA) was used to measure the antibody level of immunized laying hens, the recombinant antigen was diluted with coating solution, added to the ELISA plate at 0.1ug / well, and coated overnight at 4°C. Pour off the coating solution and wash 5 times with PBST, then blot dry with absorbent paper; block the plate with PBS containing 3% BSA, 200ul per well, incubate at 37°C for 1 hour and wash as before; dilute the IgY antibody to be tested in a 2-fold gradient Add 100ul of antibody diluent to each well, incubate at 37°C for 2 hours and wash as before; add 100ul II anti-HRP-labeled goat anti-chicken IgG to each well at a dilution of 1:10000 (purchased from Promega, G1351), and incubate at 37°C for 2 hours After 1 hour, wash as before; then add 100ul of chromogenic solution to each well, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com