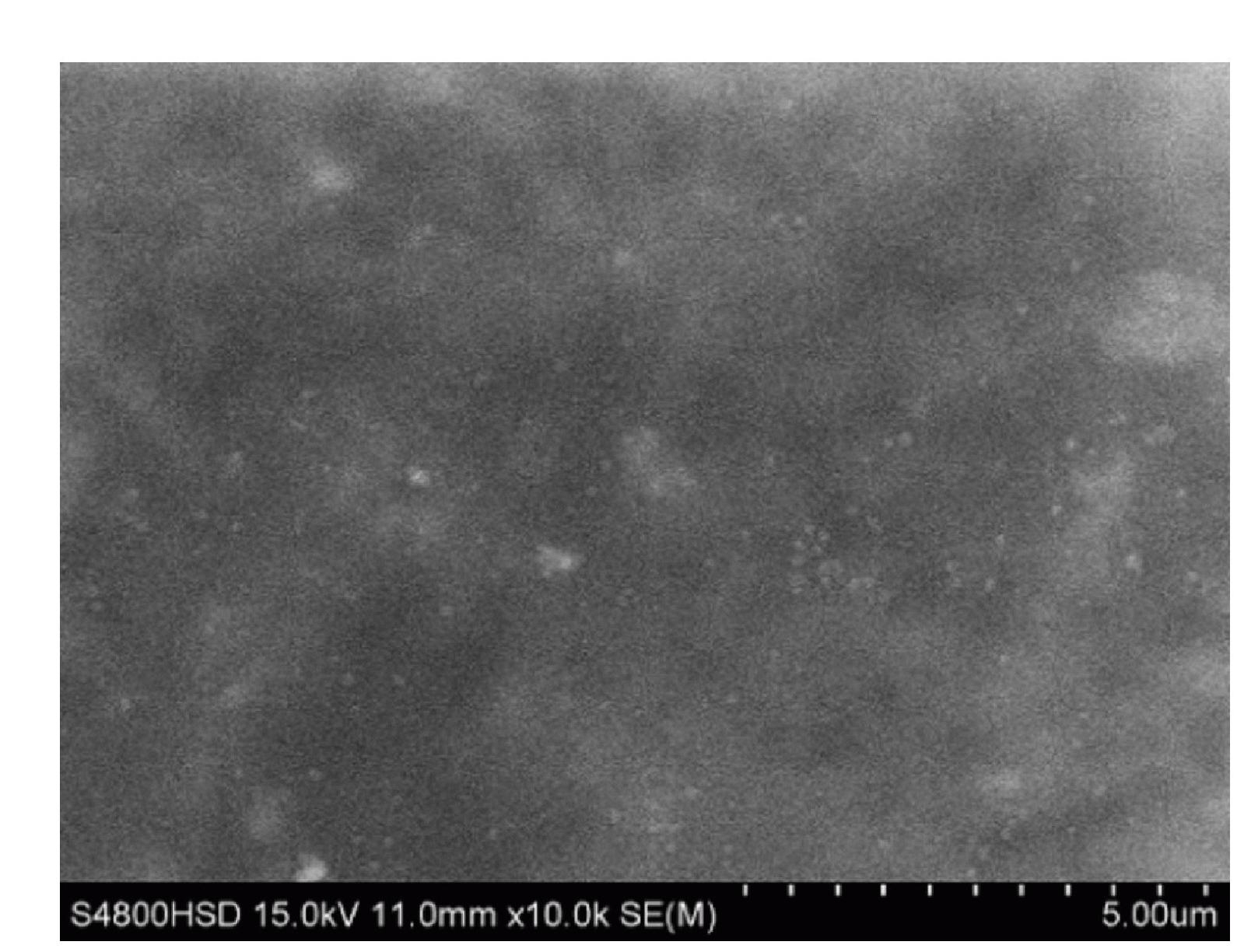
Method for electrodepositing lithium-copper alloy in ionic liquid system
An ionic liquid and electrodeposition technology, which is applied in the field of electrodeposition of lithium-copper alloys, can solve the problems of high energy consumption and difficult control of lithium element content, and achieve the effects of low energy consumption, good solubility, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0016] Specific embodiment one: the method for electrodepositing lithium-copper alloy in the ionic liquid system of the present embodiment is realized through the following steps: 1. LiBF 4 , Cu(BF 4 ) 2 Add ionic liquid 1-butyl 3-methylimidazolium tetrafluoroborate (BMIMBF 4 ), stirring and dissolving the electrolyte, in which LiBF 4 The concentration is 40~160g / L, Cu(BF 4 ) 2 The concentration is 100-200g / L, and the additive accounts for 0.5%-2% of the total mass of the electrolyte, and the additive is butynediol; 2. After the cathode material is subjected to electrodeposition pretreatment, the cathode material and the anode material are At the same time, immerse in the electrolyte, and conduct electrodeposition for 10-120 minutes in a constant current mode, that is, lithium-copper alloy is obtained on the surface of the cathode material; wherein, in step 2, the current density is controlled to be 5-10A / m 2 , the cathode material is copper, nickel, titanium or stainless...
specific Embodiment approach 2
[0022] Embodiment 2: The difference between this embodiment and Embodiment 1 is that the LiBF in step 1 4 The concentration is 80~120g / L. Other steps and parameters are the same as those in Embodiment 1.
specific Embodiment approach 3
[0023] Embodiment 3: The difference between this embodiment and Embodiment 1 is that the LiBF in step 1 4 The concentration is 100g / L. Other steps and parameters are the same as those in Embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com