Bi-dihydroxy ethylene urea, derivatives, preparation method and application thereof
A bis-dihydroxyethylene urea and bis-dihydroxyethylene technology, which is applied in the field of synthesis and purification of bis-dihydroxyethylene urea and its various derivatives, can solve problems such as human injury, environmental pollution, and restrictions on the use of cross-linking agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
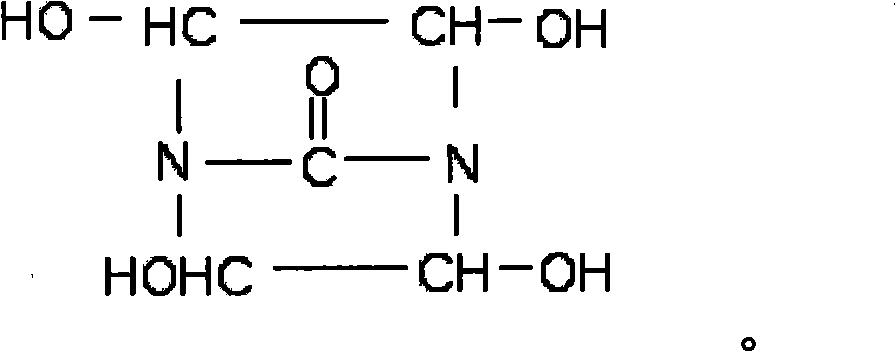
[0023] Synthesis of Dihydroxyethylene Urea
[0024] With 60g urea, 290g40% glyoxal (the molar ratio of glyoxal and urea is 2: 1), 1.5gNaOH is sequentially added in the reaction three-necked flask respectively, starts the reaction stirrer, fills the air in the reaction three-necked flask with nitrogen replacement, Under the protection of nitrogen, raise the temperature to 60±2°C, react for 2 hours, then raise the temperature to 80-90°C, and react for 4 hours to prepare an aqueous solution of bisdihydroxyethylene urea with a solid content of 50%. Add 50 g of sulfonic acid type polystyrene ion exchange resin to 50% bisdihydroxyethylene urea aqueous solution, stir for 1 hr, then filter the ion exchange resin with filter paper to remove a small amount of NaOH in the product. Obtain a pure bis-dihydroxyethylene urea aqueous solution, and finally use a vacuum rotary evaporator at 90±2°C to remove the solvent water in the product, and then obtain a light yellow transparent liquid bis-...
Embodiment 2
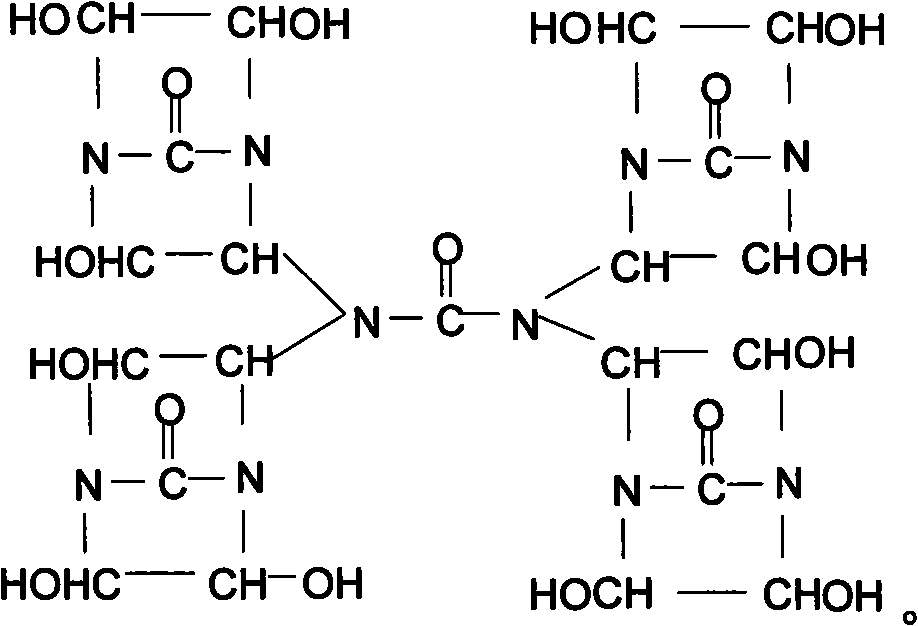
[0026] Synthesis of bisdihydroxyethylene urea derivative I (chemical formula (2))
[0027] Add 15g urea in the 50% double dihydroxyethylene urea aqueous solution 350g that contains Naoh catalyst that embodiment 1 prepares, in N 2 Under protection, raise the temperature to 90±2°C, stir and react for 4 hours to obtain a 52.3% derivative I aqueous solution, add 50 g of sulfonic acid polystyrene ion exchange resin to the product aqueous solution, stir at room temperature for 1 hour, and remove NaOH in the solution , use filter paper to filter out the ion exchange resin to obtain a pure aqueous solution of derivative I, and use a vacuum rotary evaporator to remove the solvent water in the product under the condition of 90±2°C to obtain a light yellow transparent viscous liquid derivative 1, the product yield is more than 98%.
Embodiment 3
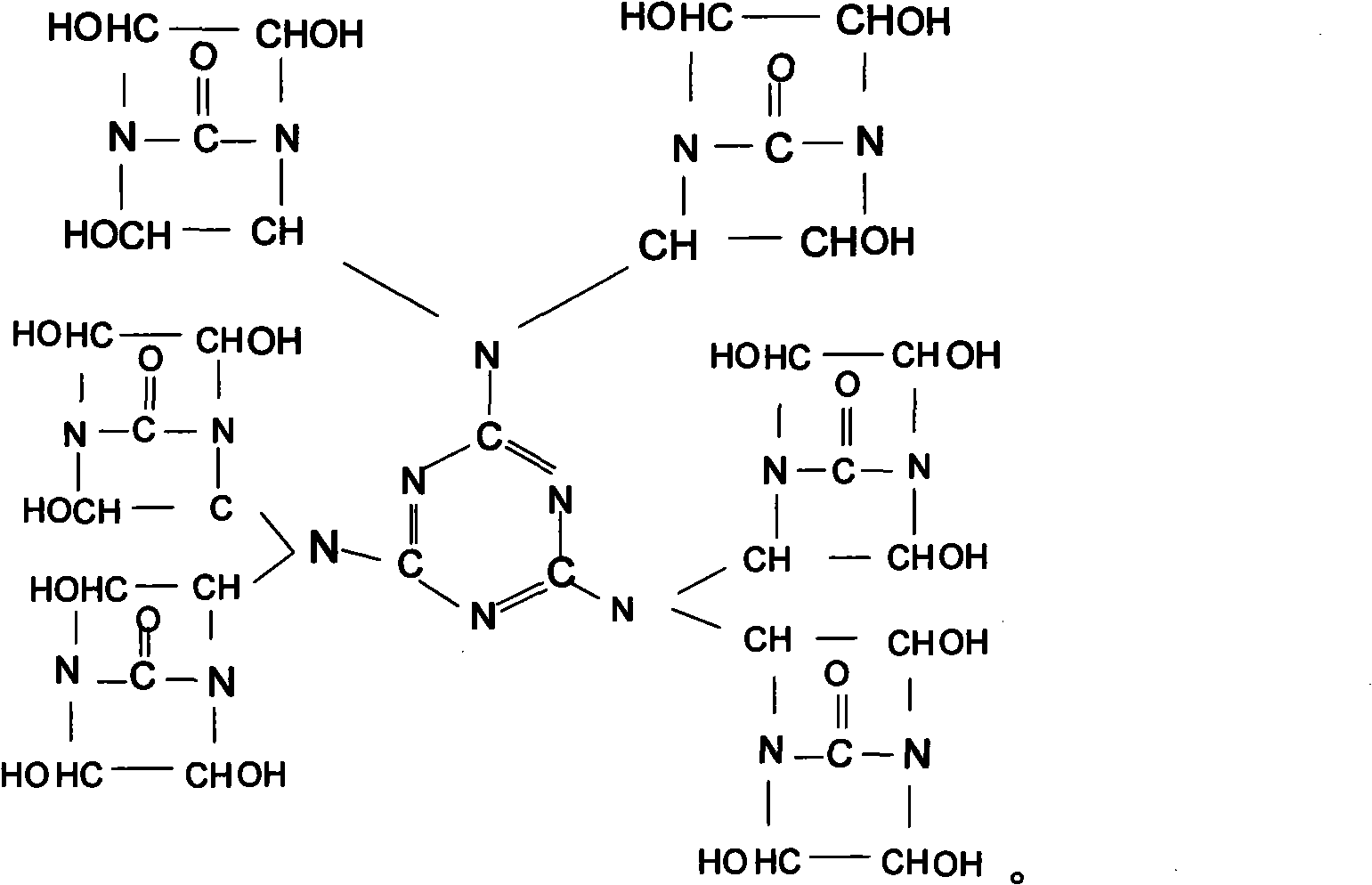
[0029] Synthesis of Bisdihydroxyethylene Urea Derivative II (Chemical Formula (3))
[0030] Add melamine 21g in the 50% double dihydroxyethylene urea aqueous solution 350g that contains NaOH that embodiment 1 prepares, in N 2 Under protection, heat up to 90±2°C, stir and react for 4 hours to obtain a 53.3% derivative II aqueous solution, add 50 g of sulfonic acid styrene ion exchange resin to this aqueous solution, stir at room temperature for 1 hour, remove NaOH in the solution, and then Use filter paper to filter out the ion exchange resin to obtain a pure aqueous solution of derivative II. Use a vacuum rotary evaporator at 90±2°C to remove the solvent water in the product to obtain a yellow, transparent, viscous liquid derivative II, the product yield is above 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com