Andrographolide derivative and preparation method and application thereof
A technology of andrographolide and derivatives, applied in the directions of drug combination, metabolic diseases, antibacterial drugs, etc., can solve problems such as indirect causes of antitumor activity, and achieve obvious effect and hypoglycemic effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Synthesis of Example 1 Compound 2
[0067] Andrographolide 1 (150mg, 0.43mmol) was dissolved in 2,2-dimethoxypropane (0.2ml, 0.85mmol), pyridinium p-toluenesulfonate (3mg, 0.88mmol) and toluene / DMSO (3ml / 0.4ml) of the mixed solution, stirred at 80°C for 1 hour, after the reaction was complete, cooled to room temperature, and the reaction mixture was terminated with triethylamine (0.1ml). The reaction mixture was diluted with toluene (20ml), washed with water (15ml), and the organic layer was washed with anhydrous Na 2 SO 4 After drying and concentration, the resulting white solid was washed with ether and filtered to obtain compound 2 (130 mg, 78%).
Embodiment 2
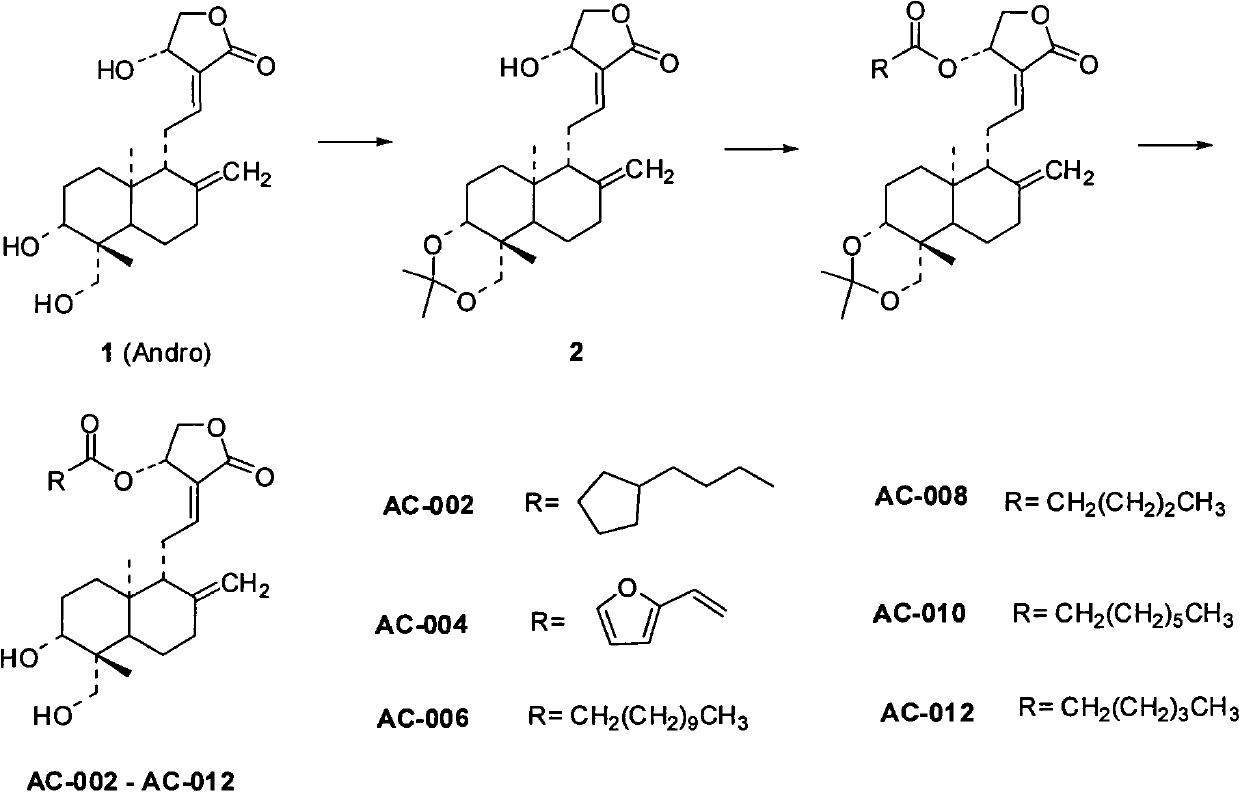
[0068] Example 2 Synthesis of compound AC-002 (synthetic route diagram as figure 1 shown)
[0069] Ethyl chloroformate (0.33ml, 3.4mmol) was added to 5-cyclopentylpentanoic acid (350mg, 2.0mmol) CH 2 Cl 2 (16ml) solution, add 0.63mL of triethylamine, and stir in an ice-water bath under nitrogen protection for 1h. Compound 2 (200 mg, 0.5 mmol) was dissolved in CH 2 Cl 2 (20ml), was added dropwise to the mixture, and stirred at room temperature for 40h. Add CH after reaction 2 Cl 2 (50ml) diluted with NaHCO 3 Aqueous solution and water wash. Anhydrous NaSO for organic layer 4 Dry and concentrate in vacuo. The mixture was separated on a silica gel column (PE:EA=2:1) to obtain compound AC-001 as a colorless viscous liquid (132 mg, 47.6%).
[0070] Compound AC-001 was added to acidic aqueous solution (AcOH / H 2 O=7 / 3, 10ml), stirred at room temperature within 30min. The reaction mixture was added with water and NaHCO 3 , with a large amount of CH 2 Cl 2 extraction....
Embodiment 3
[0073] Embodiment 3. the synthesis of compound AC-004 (synthetic route figure such as figure 1 shown)
[0074] Ethyl chloroformate (0.33ml, 3.4mmol) was added to 3-furanoacrylic acid (283mg, 2.0mmol) CH 2 Cl 2 (16ml) solution, add 0.63mL of triethylamine, and stir in an ice-water bath under nitrogen protection for 1h. Compound 2 (200 mg, 0.5 mmol) was dissolved in CH 2 Cl 2 (20ml), was added dropwise to the mixture, and stirred at room temperature for 40h. Add CH after reaction 2 Cl 2 (50ml) diluted with NaHCO 3 Aqueous solution and water wash. Anhydrous NaSO for organic layer 4 Dry and concentrate in vacuo. The mixture was separated on a silica gel column (PE:EA=2:1) to obtain compound AC-003 as a colorless viscous liquid (112.3 mg, 43%).
[0075] Compound AC-003 was added to acidic aqueous solution (AcOH / H 2 O=7 / 3, 10ml), stirred at room temperature within 30min. The reaction mixture was added with water and NaHCO 3 , with a large amount of CH 2 Cl 2 extrac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com