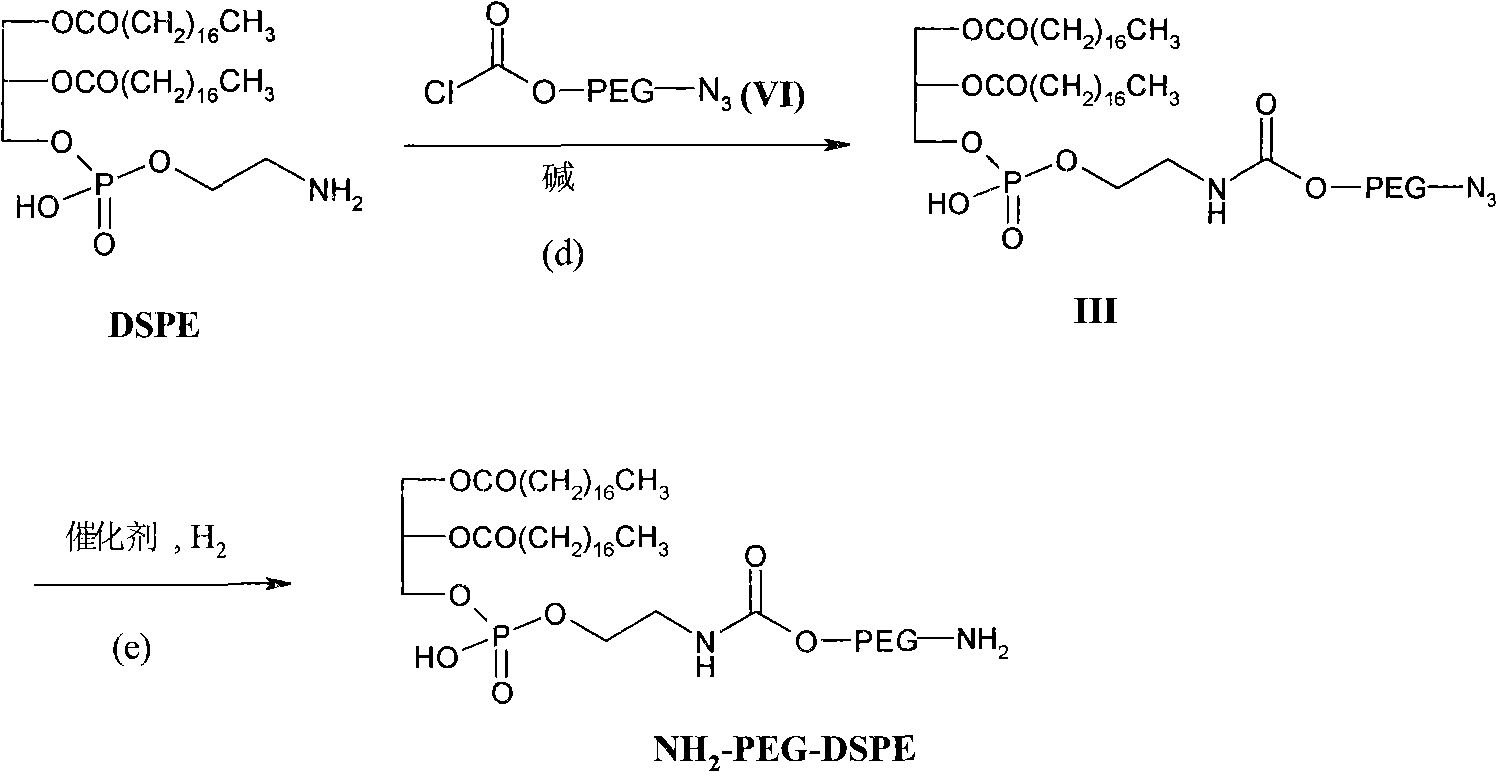
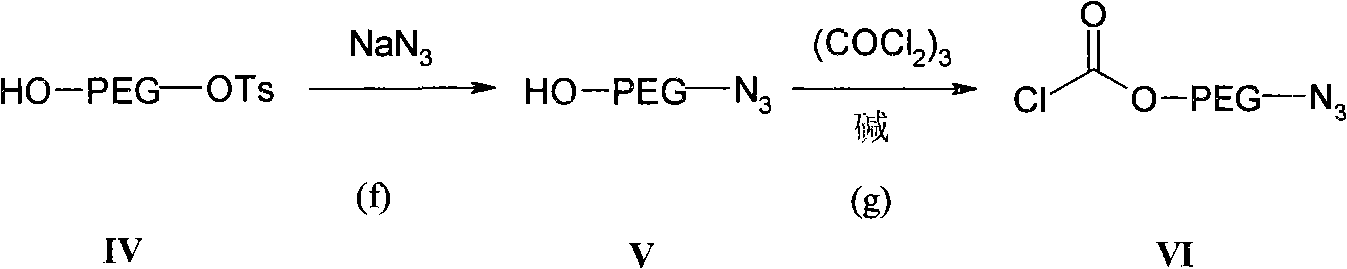Preparation methods of distearoyl phosphatidyl ethanolamine and amino polyethylene glycol derivatives thereof
A technology of distearoylphosphorylethanol and stearoylphosphorylethanolamine, which is applied in the field of preparation of distearoylphosphorylethanolamine and its amino pegylated derivatives, which can solve the difficulty of tracking the reaction process and low temperature requirements It is easy to operate, reduce the cost of raw materials, and achieve the effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0029] The preparation of embodiment 1.2-hydroxyethylcarbamate benzyl ester
[0030] Mix 12mL of ethanolamine in 300mL of dioxane and water mixed solvent (1:1), add 100mg of N,N-dimethylaniline, 32mL of benzyloxycarbonyl chloride and 28mL of triethylamine respectively, and stir the mixture at room temperature for 12h , and the solvent was removed under reduced pressure to obtain a viscous solution. It was diluted with 200 mL of dichloromethane and 100 mL of water, the organic layer was separated, and the aqueous layer was extracted with dichloromethane (50 mL×3). The organic layers were combined, washed twice with 0.1M aqueous hydrochloric acid, water, and saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain 37.0 g of a white crude product. The obtained crude product was heated and dissolved with 65 mL of petroleum ether / ethyl acetate mixed solvent (40 mL of ethyl acetate, 25 mL of petroleum ether) to obtain a milky white solution, which ...
Embodiment 2
[0031] Embodiment 2. Preparation of compound II
[0032] In a 1000mL dry three-necked flask with a thermometer inserted, add 250mL of dry anhydrous tetrahydrofuran under nitrogen protection, add 3.6g of triethylamine after cooling to 0°C, and then slowly dropwise add 0.34g of POCl 3 50mL of anhydrous tetrahydrofuran solution, kept at 0°C and continued to stir for 0.5h. Dissolve 18.6g of 1,2-distearylglyceride in 250mL of anhydrous tetrahydrofuran, and then slowly add it dropwise to the above reaction solution, controlling the temperature of the reaction solution not higher than 0°C, and the addition is completed in about 2.5h. Stirring was continued for 1 h at 0 °C, then overnight at room temperature. Below 10°C, slowly dropwise add 5.8g of benzyl 2-hydroxyethylcarbamate and 3.6g of triethylamine in anhydrous tetrahydrofuran (100mL), stir at room temperature for 24h, then add 15mL of water to terminate the reaction , the organic solvent was evaporated under reduced pressure,...
Embodiment 3
[0034] The preparation of embodiment 3.DSPE
[0035] Add 1.9g II to 600mL ethanol / dichloromethane (1:1) mixed solvent, heat to 50°C to obtain a colorless and clear solution, then add 0.5g 10% palladium carbon, under 10 atmospheres of hydrogen, at 50°C Reaction 4h. After the reaction was completed, it was filtered while it was hot, and the filtrate was concentrated to 150 mL, a white solid was precipitated, cooled and left for 3 hours, and filtered to obtain a crude product. Recrystallized with ethanol / dichloromethane mixed solvent to obtain 1.3 g of white solid (yield 82%).
[0036] IR (v, cm -1 ): 3263s(-NH), 2917vs(Alkyl), 1737vs(O=C-O), 1627s(-NH), 1265vs(COO-C); ESI-MS(m / z): 746[M-H] - ,748[M+H] + ; 1 H-NMR (300MHz, CDCl 3 ): δ=5.20-5.17 (m, 1H, CH 2 -C H -CH 2 ), 4.12-3.99 (m, 4H, O-C H 2 -C H 2 -O), 3.48-3.27(m, 2H, OP-OC H 2 ), 3.14-3.08 (m, 2H, OP-OC H 2 ), 2.30 (br, 4H, (C H 2 CO) 2 ), 1.58 (br, 4H, (C H 2 CH 2 CO) 2 ), 1.41-1.12 (m, 56H, alkyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com