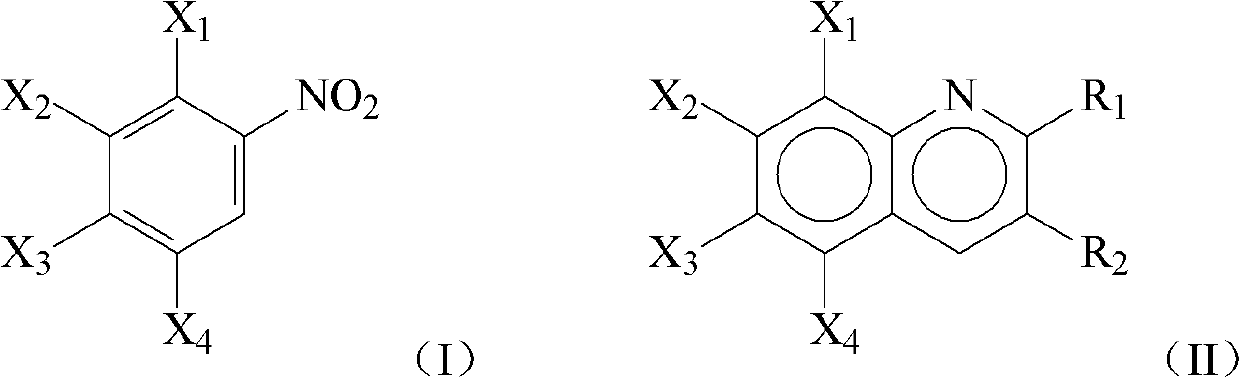
Method for one-pot liquid phase synthesis of quinoline and derivative thereof
A liquid-phase synthesis and derivative technology, applied in the direction of organic chemistry, can solve the problems of difficult catalyst separation and recovery, high reaction temperature, high equipment requirements, etc., to avoid easy oxidation and deterioration and storage, less equipment investment, and reduce production cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (x wt%)Cu-(y wt%)Pd / Al 2 o 3 (x wt% and ywt% are the loadings of Cu and Pd respectively) The preparation process of the catalyst is as follows: use deionized water to make a certain amount of carrier into a slurry at a temperature of 70-90 ° C, and then drop a certain amount of H 2 PdCl 4 (0.05gPd / ml) and Cu(NO 3 ) 2 Aqueous solution, maintain the temperature range and fully stir and impregnate for 5 hours, then use 10% NaOH (mass percentage) aqueous solution to adjust the pH value of the solution to 8.6-10, continue to age for 30 minutes, and then cool to room temperature, filter, and wash the filter cake with deionized water to medium and dried at 110°C for 5 hours in an air atmosphere, then calcined at 260°C for 4 hours in an air atmosphere, and finally raised to 260°C at a rate of 1°C / min in a hydrogen flow of 100mL / min, and reduced at a constant temperature at 260°C 2h, and finally lowered to room temperature in a hydrogen atmosphere to obtain the catalyst.
Embodiment 2 9
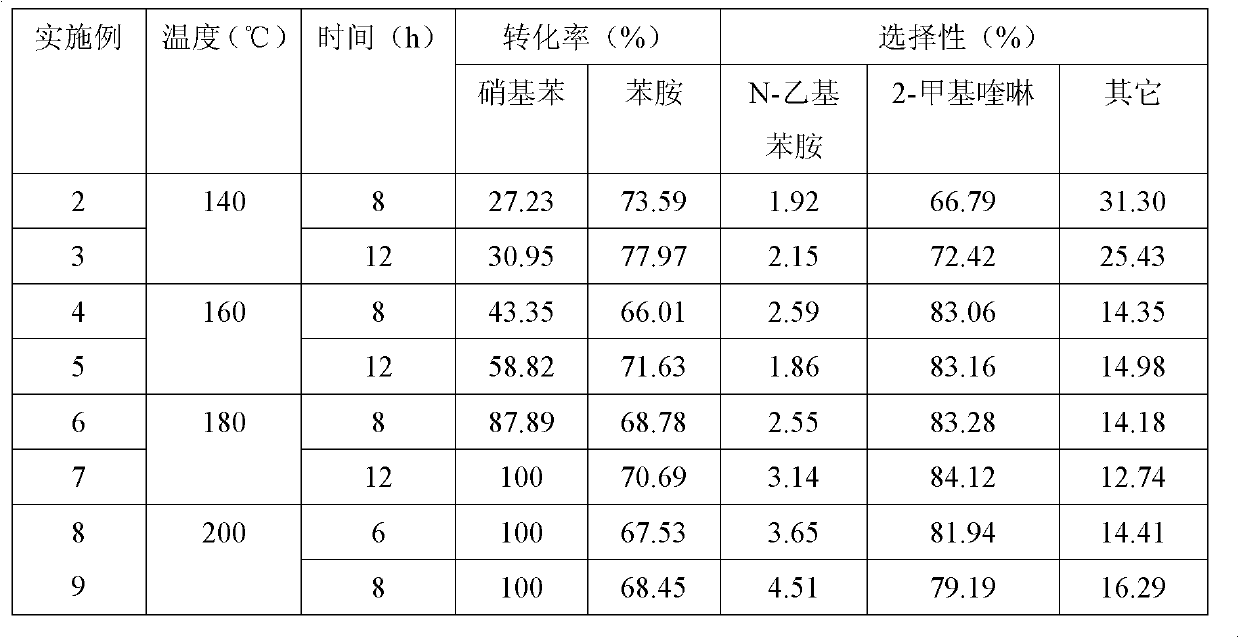
[0030] Embodiments two to nine investigated the influence of reaction temperature on the reaction performance of nitrobenzene and ethanol to synthesize 2-methylquinoline. In a 500mL stainless steel autoclave, add 10mL of nitrobenzene, 60mL of absolute ethanol, 20mL of water, and Cu 5%-Pd 5% / Al prepared in Example 1 2 o 3 Catalyst 0.5g, close the reactor, replace the air in the reactor with nitrogen and keep the pressure in the reactor, after warming up to the set reaction temperature, adjust the reaction pressure to be 1.0MPa, start stirring (800r / min), constant temperature After reacting for a certain period of time, the reaction was stopped and cooled to room temperature, the reaction solution was taken out, the catalyst was removed by filtration, and the filtrate was analyzed by gas chromatography. The experimental results are shown in Table 1.
[0031] The experimental result of the influence of table 1 reaction temperature on the synthetic 2-methylquinoline of nitrobenz...
Embodiment 61
[0058] In a 10L stainless steel autoclave, add 825mL of nitrobenzene, 3300mL of absolute ethanol, 1650mL of water and Cu 5%-Pd 5% / Al prepared in Example 1 2 o 3 Catalyst 55g, close the reactor, replace the air in the reactor with nitrogen and maintain the pressure in the reactor, raise the temperature to 180°C, adjust the reaction pressure to 3.5MPa, start stirring (800r / min), keep the constant temperature reaction for 19.5h, stop React and be cooled to room temperature, take out reaction liquid, filter and remove catalyst, filtrate is analyzed by gas chromatography, 2-methylquinoline selectivity 63.5%, the selectivity of N-ethylaniline 0.7%, aniline selectivity 27.1%, other by-products The product is 8.7%, and the conversion rate of nitrobenzene is 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com