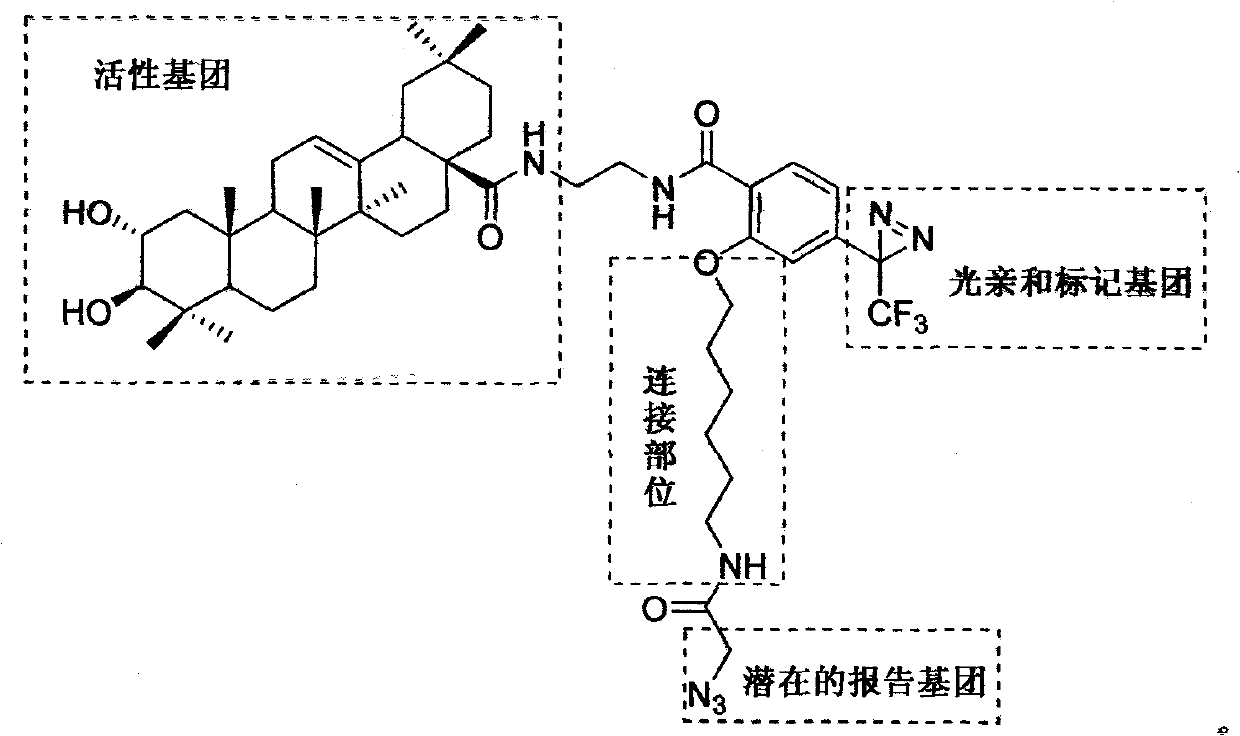
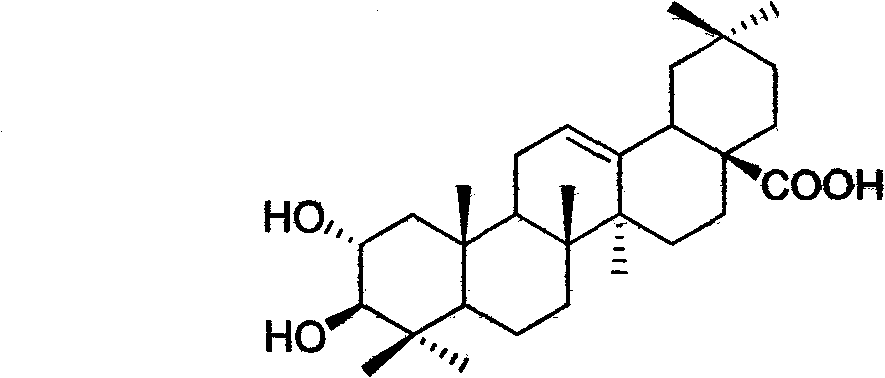
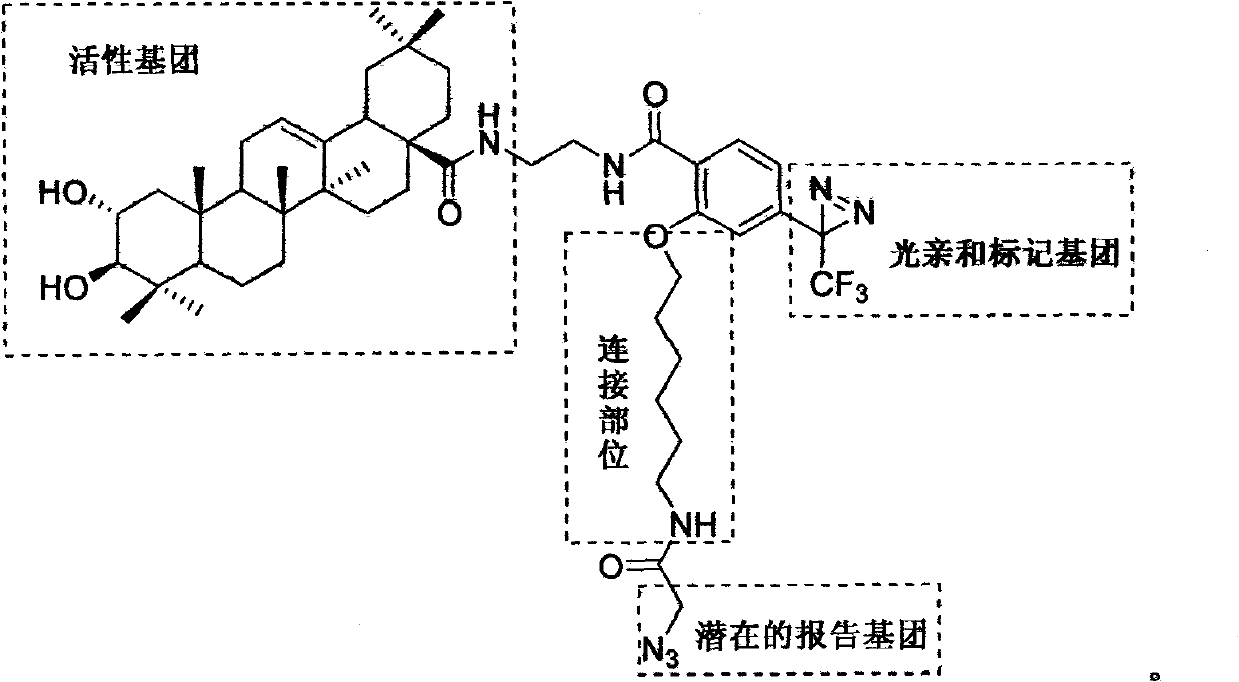
Light affinity labelling small molecular probe based on maslinic acid and preparation method thereof
A technology of small molecule probes and labeled probes, applied in the production of steroids, organic chemistry, bulk chemicals, etc., can solve problems such as unsolvable problems, and achieve the effect of promoting research and development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The first step is the preparation of the first compound I
[0033] Dissolve 3.0 g of maslinic acid in 50 mL of N, N-dimethylformamide, add 4.1 mL of acetic anhydride, add 10 mL of pyridine, react at room temperature for 6 hours, evaporate the solvent under reduced pressure, add 20 mL of water, and extract with dichloromethane 3 times, each Use 50 mL each time, combine the organic phases, wash with 10% dilute hydrochloric acid twice, each time using 20 mL, then wash twice with saturated sodium bicarbonate solution, each time using 20 mL, dry over anhydrous sodium sulfate, evaporate the solvent under reduced pressure, Silica gel column chromatography, the eluent is a mixed solvent of petroleum ether and ethyl acetate, and the ratio of the mixed solvent is petroleum ether:ethyl acetate=3:1, to obtain 3.2g of the first compound I, with a yield of 91%;
[0034] The preparation of the second step second compound II
[0035] Dissolve 2.7g of the first compound I in 50mL of di...
Embodiment 2
[0044] The first step is the preparation of the first compound I
[0045] Dissolve 3.0g of maslinic acid in 50mL of dichloromethane, add 3.5mL of acetyl chloride, add 10mL of triethylamine, react at room temperature for 6 hours, evaporate the solvent under reduced pressure, add 20mL of water, extract with ethyl acetate three times, each time with 50mL, The organic phases were combined, washed twice with 10% dilute hydrochloric acid, 20 mL each time, washed twice with 20 mL each time with saturated sodium bicarbonate solution, dried over anhydrous sodium sulfate, evaporated to remove the solvent under reduced pressure, and subjected to silica gel column chromatography , the eluent is a mixed solvent of petroleum ether and ethyl acetate, and the ratio of the mixed solvent is petroleum ether: ethyl acetate=3: 1 to obtain 3.3g of the first compound I, with a yield of 94%;
[0046] The preparation of the second step second compound II
[0047] 2.7g of the first compound I was diss...
Embodiment 3
[0056] The first step is the preparation of the first compound I
[0057] Dissolve 3.0g of maslinic acid in 50mL of tetrahydrofuran, add 3.5mL of acetyl chloride, add 10mL of triethylamine, react at room temperature for 6 hours, distill off the solvent under reduced pressure, add 20mL of water, extract 3 times with 30mL of chloroform, combine the organic phases, Wash 2 times with 10% dilute hydrochloric acid, 20 mL each time, then wash 2 times with 20 mL each time with saturated sodium bicarbonate solution, dry over anhydrous sodium sulfate, evaporate the solvent under reduced pressure, silica gel column chromatography, eluent It is a mixed solvent of petroleum ether and ethyl acetate, the ratio of the mixed solvent is petroleum ether:ethyl acetate=3:1, and 3.0 g of the first compound I is obtained, and the yield is 85%;
[0058] The preparation of the second step second compound II
[0059] Dissolve 2.7 g of the first compound I in 40 mL of chloroform, add 10 ml of thionyl c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com