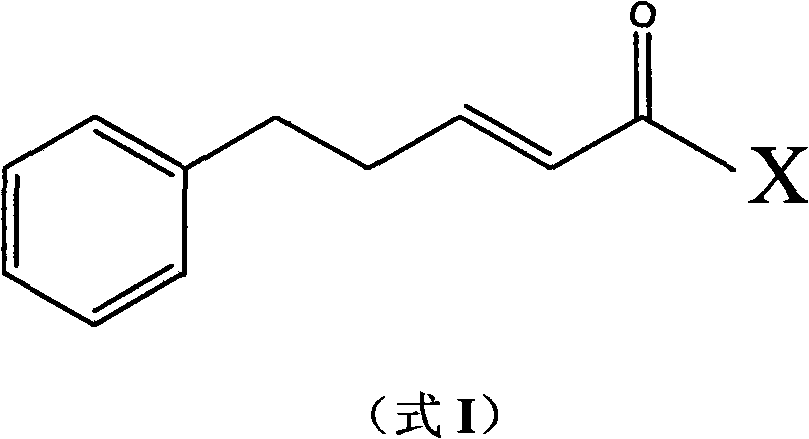
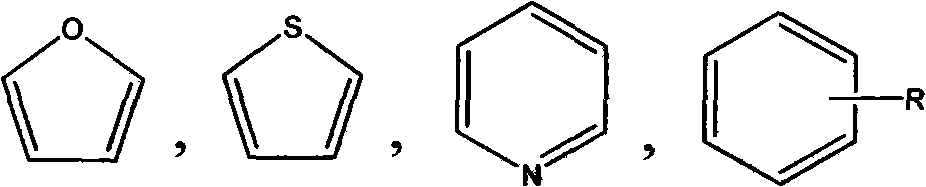
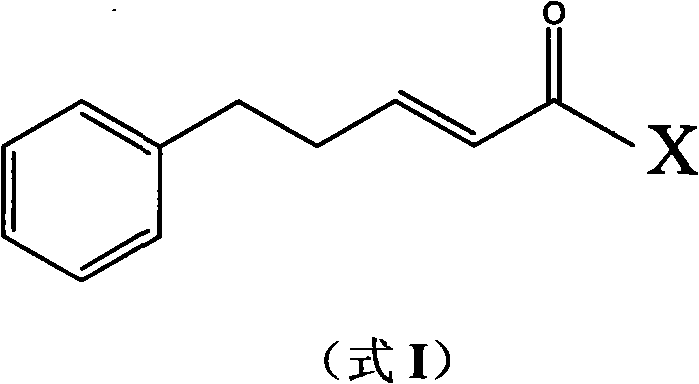
(E)-1-aryl-5-phenyl-2-alkene-1-pentanone compounds and synthesis and application thereof
A compound, aryl ethyl ketone technology, applied in (E)-1-aryl-5-phenyl-2-ene-1-pentanone compound and its synthesis and application fields, can solve the problem of unclear active ingredients and other problems, to achieve the effect of simple product purification method, simple chemical structure and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the preparation of 2-bromo-1-(pyridin-2-yl)ethanone, its synthetic route is as follows:
[0031]
[0032] Put 2-acetylpyridine (18.15 g, 0.15 mol) in a 250 ml three-neck flask, add 100 ml of diethyl ether, and stir well. Bromine (24 g, 0.15 mol) was slowly added dropwise from the dropping funnel, and after the dropwise addition was completed, it was stirred at room temperature for 2 h. 80ml of water was added, the organic phase was separated, washed with saturated brine, dried over anhydrous magnesium sulfate, and 24.54g of white blocky crystals were recrystallized from absolute ethanol. Yield 82.2%.
Embodiment 2
[0033] Embodiment 2: the preparation of (2-oxygen-2-(pyridin-2-yl) ethyl) triphenylphosphine bromide, its synthetic route is as follows:
[0034]
[0035]Put triphenylphosphine (2.62g, 10mmol) in a 250ml three-neck flask, add 100ml THF, and heat to reflux. A THF solution of 2-bromo-1-(pyridin-2-yl)ethanone (1.99 g, 10 mmol) was added dropwise. After completion, the reaction system was refluxed for 4h. Cool to room temperature, filter, and wash with anhydrous ether to obtain 2.02 g of white powder. Yield 45.7%.
Embodiment 3
[0036] Embodiment 3: the preparation of phosphine, its synthetic route is as follows:
[0037]
[0038] Put (2-oxo-2-(pyridin-2-yl)ethyl)triphenylphosphine bromide (2.02g, 4.38mmol) in a 250ml three-necked flask, add 30ml methanol, 30ml water, stir to dissolve . Another 60 ml of 2N aqueous sodium hydroxide solution was added. Stir overnight at room temperature. It was extracted with chloroform, and the organic layer was washed with saturated brine. Dry over anhydrous sodium sulfate. Filtration and rotary evaporation gave 1.21 g of white powder with a yield of 72.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com