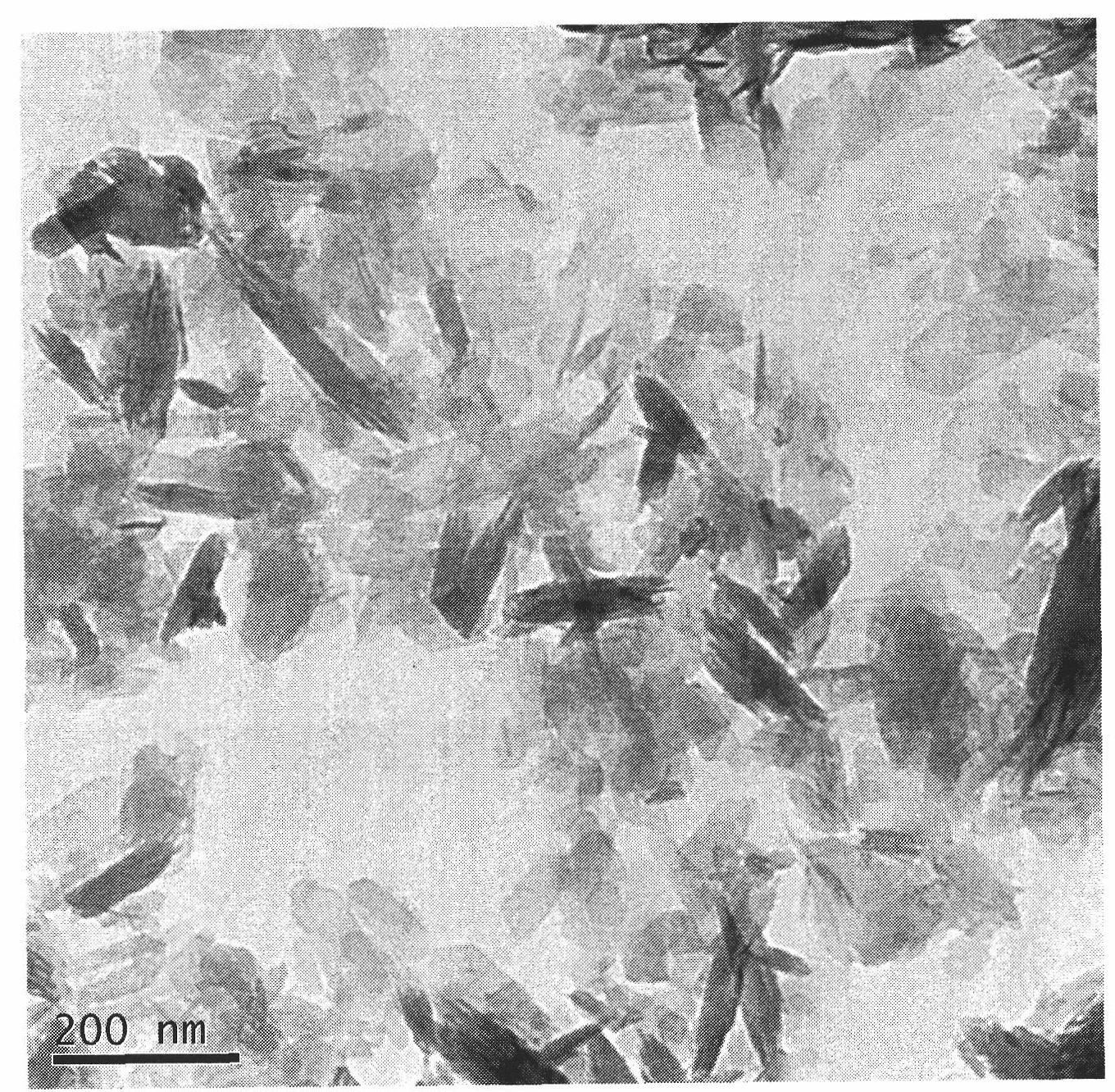
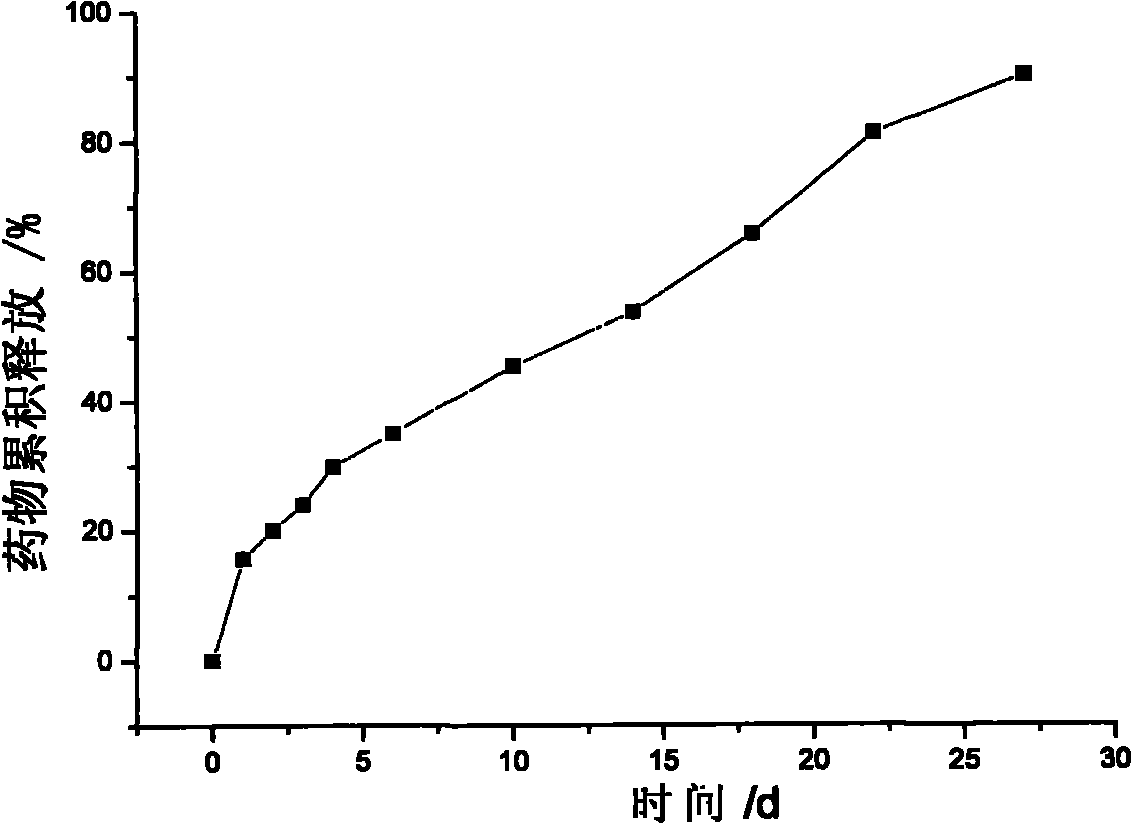
Gentamicin sulfate wrapped in polylactic acid/nano-hydroxyapatite composite microspheres and preparation method thereof
A technology of nano-hydroxyapatite and gentamicin sulfate, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas to overcome short sustained release time and particle size dispersion Uniform, long-term drug release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0025] Preparation of PLA / nHA-GS composite microspheres: ①Preparation of nHA-GS: Add 0.6g HA to 6ml aqueous solution containing 120mgGS, first mix with 60kHz ultrasonic, then magnetically stir for 24h, freeze-dry at -40°C and heat up to 10°C, The white lyophilized powder nHA-GS was obtained. ②Preparation of microspheres: Add nHA-GS lyophilized powder into 15ml dichloromethane solution dissolved with 1.5g PLA, stir at 8000rpm for 1min, mix and disperse by ultrasonic to form S / O emulsion; ③Add the above S / O emulsion Quickly pour into 750ml deionized aqueous solution dissolved with 1.2g emulsifier methyl cellulose to obtain S / O / W emulsion, and magnetically stir at 400rpm for 4h to completely volatilize dichloromethane; after standing for 4h, centrifuge at 10000rpm , the precipitate was separated, washed three times with deionized water, and then freeze-dried at -40°C to obtain the product of the present invention, which was refrigerated at -4°C. The drug loading capacity of the ...
example 2
[0030] Preparation of PLA / nHA-GS composite microspheres: ① Preparation of nHA-GS: Add 0.6g HA to 6ml aqueous solution containing 60mgGS, first mix with 80kHz ultrasonic, then magnetically stir for 24h, freeze-dry at -40°C and heat up to 10°C. The white lyophilized powder nHA-GS was obtained. ②Preparation of microspheres: Add nHA-GS freeze-dried powder into 15ml of acetone solution dissolved with 1.5g PLA, stir at 4000rpm for 1min at high speed, and form S / O emulsion after fully mixing and dispersing; ③Pour the above S / O emulsion quickly Dissolve 0.6g of emulsifier methylcellulose in 300ml of deionized aqueous solution to obtain an S / O / W emulsion, and stir it magnetically at 800rpm for 4h to make the acetone evaporate completely; after standing for 4h, centrifuge at a speed of 10000rpm to separate Precipitated, washed three times with deionized water, then freeze-dried at -40°C and warmed to 10°C, and the obtained microspheres were refrigerated at -4°C. The drug loading capaci...
example 3
[0032]Preparation of PLA / nHA-GS composite microspheres: ①Preparation of nHA-GS: Add 0.2g HA to 10ml aqueous solution containing 120mgGS, first mix with 40kHz ultrasonic, then magnetically stir for 24h, freeze-dry at -40°C and heat up to 10°C, The white lyophilized powder nHA-GS was obtained. ②Preparation of microspheres: Add nHA-GS freeze-dried powder into 15ml chloroform solution dissolved with 1.5g PLA, stir at 2000rpm at high speed for 1min, mix well and disperse to form S / O emulsion, ③Pour the above S / O emulsion quickly Dissolve 0.6g of emulsifier methyl cellulose in 300ml of deionized aqueous solution to obtain an S / O / W emulsion, stir it magnetically at 1000rpm for 4h, and let the chloroform volatilize completely; after standing for 4h, centrifuge at a speed of 10000rpm to separate Precipitated, washed three times with deionized water, then freeze-dried at -40°C and warmed to 10°C, and the obtained microspheres were refrigerated at -4°C. The drug loading capacity of the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com