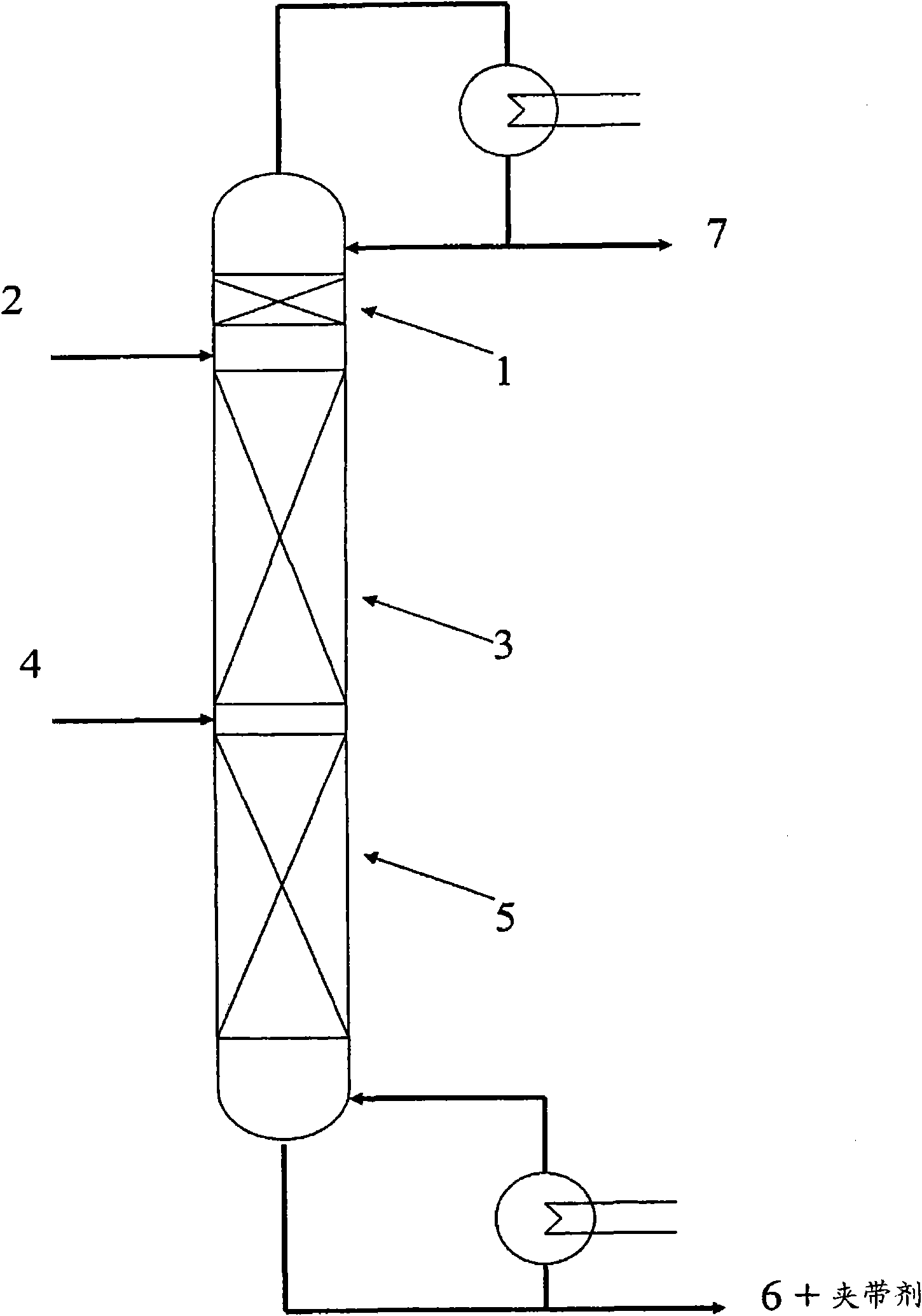
Process for the separation of fluorocarbons using ionic liquids
A technology of ionic liquids and mixtures, applied in chemical instruments and methods, organic chemistry, halogenated hydrocarbon preparation, etc., can solve the problems of difficult separation, inability to achieve, high investment cost and production cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0189] Separation of 1,1,2,2-tetrafluoroethane (R-134) and 1,1,1,2-tetrafluoroethane (R-134a)
[0190] This example focuses on the thermodynamic properties at infinite dilution. Pair in [bmim][PF 6 ] and [emim][Tf 2 N] in R-134 and R-134a, analysis of the activity coefficient γ at infinite dilution.
[0191] R-134 and R-134a in [bmim][PF 6 ] and [emim][Tf 2 The experimental solubility (PTx) data in N] are summarized in Examples 3 and 4 (for [bmim][PF 6 ]) and Examples 5 and 10 (for [emim][Tf 2 N]). Data have been corrected using a nonrandom two-liquid (NRTL) solution model. NRTL (S.I., Sandler, "Chemical and Engineering Thermodynamics" 3rd Edition (1999), John Wiley and Sons, Inc., New York, Chapter 7) activity coefficient (γ i ) model is given by:
[0192] ln γ 1 = x 2 2 [ τ 21 ( ...
Embodiment 2
[0221] Separation of mixtures containing 1,1,1,2-tetrafluoroethane and 1,1,2,2-tetrafluoroethane .
[0222] Using Aspen Plus TM (Aspen Technology, Inc., version 13.2, Cambridge, MA) process simulator, for the use of [emim][Tf 2 N] as extractant, separated by extractive distillation containing 1,1,1,2-tetrafluoroethane (also known as HFC-134a or R-134a) and 1,1,2,2-tetrafluoroethane ( Also known as HFC-134 or R-134) mixtures are simulated.
[0223] Think of ionic liquids as non-dissociated liquids with extremely low vapor pressures. The nonrandom two-liquid (NRTL) activity coefficient model (S.I., Sandler, "Chemical and Engineering Thermodynamics", 3rd edition (1999), John Wiley and Sons, Inc., New York, Chapter 7) was used to simulate the relationship between ionic liquids and Liquid-phase interactions between fluorocarbons and the vapor phase modeled using the Peng-Robinson equation of state. Binary NRTL interaction parameters of ionic liquids with fluorocarbons...
Embodiment 3
[0232] 1,1,2,2-tetrafluoroethane (R-134) in 1-butyl-3-methylimidazolium hexafluorophosphate [bmim][PF 6 ] Solubility in
[0233] Solubility studies were carried out at temperatures ranging from about 10°C to 75°C and pressures ranging from 0.1 bar (0.01 MPa) to about 3.5 bar (0.35 MPa), where R-134 was determined using a gravimetric microbalance in [bmim][PF 6 ] Solubility (x) or mole fraction in ]. Table 4 provides T, P and x data, respectively.
[0234] Table 4
[0235] T(℃) P(bar) R-134(mol fraction)
[0236] 10.01 0.10 0.029
[0237] 9.97 0.50 0.176
[0238] 9.99 1.00 0.357
[0239] 9.99 1.50 0.528
[0240] 9.97 2.00 0.686
[0241] 9.98 2.50 0.814
[0242] 9.96 3.00 0.974
[0243] 25.02 0.10 0.024
[0244] 24.93 0.50 0.116
[0245] 24.89 1.00 0.225
[0246] 24.93 1.50 0.330
[0247] 24.92 2.00 0.428
[0248] 25.00 2.50 0.522
[0249] 24.90 3.00 0.611
[0250] 24.94 3.50 0.689
[0251] 49.97 0.10 0.00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com