Catalyst for reforming methane with carbon dioxide for preparing synthetic gas and preparation method thereof
A carbon dioxide and catalyst technology, applied in the field of catalyst and preparation of carbon dioxide reforming methane to synthesis gas, can solve the problems of catalyst performance differences, different preparation methods, catalyst deactivation, etc., achieve good mechanical properties and thermal stability, and cheap raw materials The effect of easy availability and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
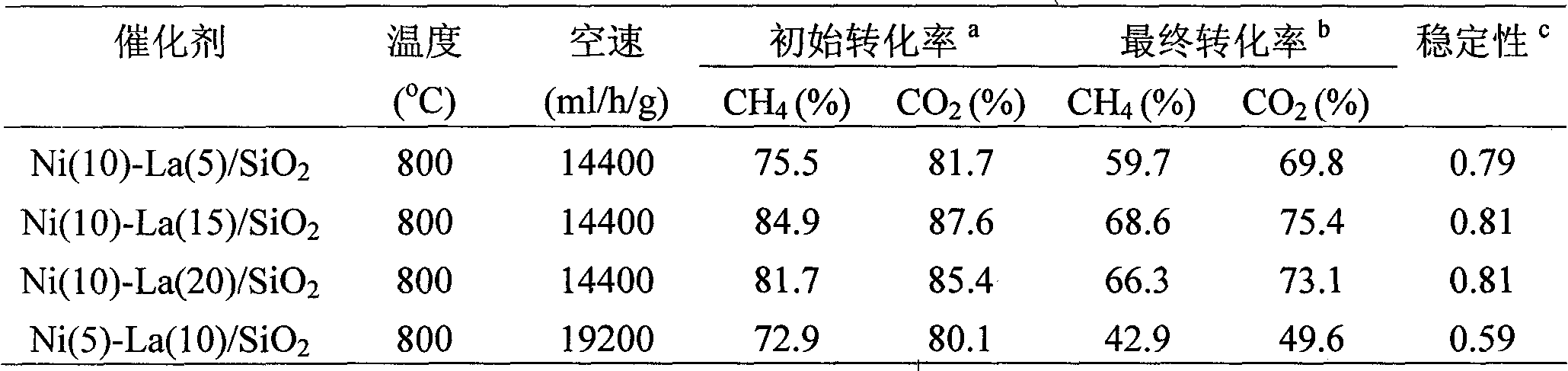
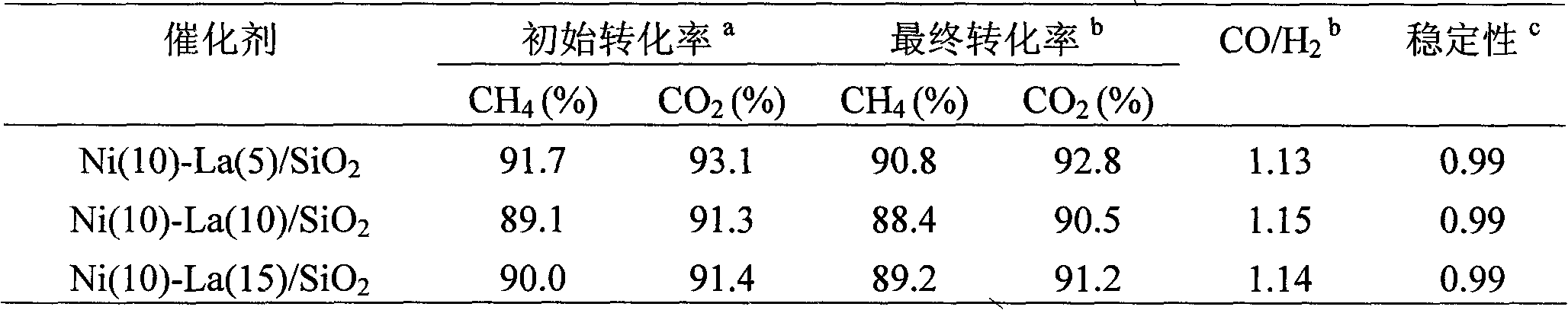
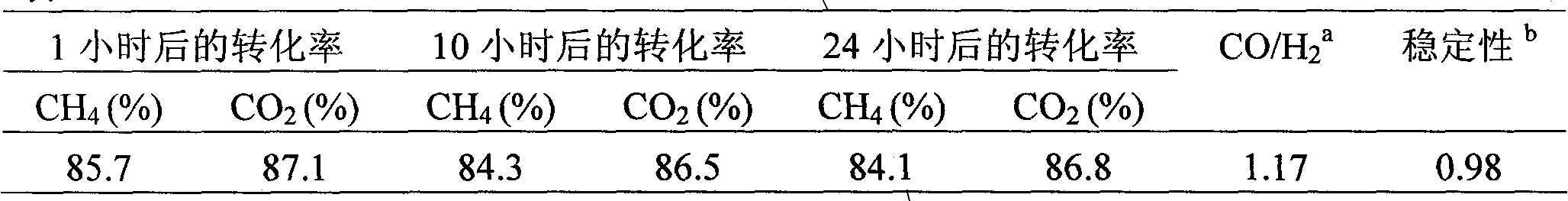
[0021] 0.9908 g Ni(NO 3 ) 2 ·6H 2 O and 0.2275 g La(CH 3 COO) 3 Dissolve in distilled water at 60°C, add 2.0 grams of 20-40 mesh SiO 2 Carrier, soaked at room temperature for 24 hours, evaporated to dryness in a water bath at 80°C, then dried at 110°C for 4 hours, and finally calcined at 800°C in a muffle furnace for 5 hours to obtain Ni(10)-La(5) / SiO 2 catalyst.
Embodiment 2
[0023] 0.9908 g Ni(NO 3 ) 2 ·6H 2 O and 0.4551 g La(CH 3 COO) 3 Dissolve in distilled water at 60°C, add 2.0 grams of 20-40 mesh SiO 2 Carrier, soaked at room temperature for 24 hours, evaporated to dryness in a water bath at 80°C, then dried at 110°C for 4 hours, and finally calcined in a muffle furnace at 800°C for 5 hours to obtain Ni(10)-La(10) / SiO 2 catalyst.
Embodiment 3
[0025] 0.9908 g Ni(NO 3 ) 2 ·6H 2 O and 0.6826 g La(CH 3 COO) 3 Dissolve in distilled water at 60°C, add 2.0 grams of 20-40 mesh SiO 2 The carrier, after immersing at room temperature for 24 hours, evaporated to dryness in a water bath at 80°C, then dried at 110°C for 4 hours, and finally calcined at 800°C for 5 hours in a muffle furnace to obtain Ni(10)-La(15) / SiO 2 catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com