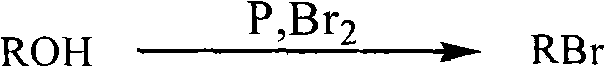Method for preparing halogenated hydrocarbons from strong acidic ionic liquid
An ionic liquid and strong acidic technology, which is applied in the preparation of halogenated hydrocarbons, chemical instruments and methods, organic chemistry, etc., can solve the problems of difficult protonation of alcoholic hydroxyl groups, long reaction time, high reaction temperature, etc., and achieve high product yield, The effect of fast reaction rate and easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] In the reaction system, add 0.2 mol of 1,7-heptanediol, 0.5 mol of 1-ethyl 3-methylimidazolium bromide [emim] Br, 0.5 mol of 1-ethyl-3-methylimidazolium bisulfate [emim] HSO 4 0.5mol, reaction temperature 60~100℃, react for 60min, after the reaction is completed, add a certain amount of water, extract the reaction solution with cyclohexane, wash and dry the organic phase with water, spin off the solvent to obtain the product 1,7-dibromo Heptane, the product yield is 93%. Add 0.5 mol of sodium bromide to the aqueous phase, stir evenly, remove water, add methanol for ion exchange, react for 24 hours, filter out unreacted sodium bromide and sodium sulfate, and spin evaporate the solvent to obtain the ionic liquid [ emim]Br is recycled.
Embodiment 2
[0059] In the reaction system, add 0.1 mol of n-octanol, 0.1 mol of 1-propyl 3-methylimidazolium chloride [pmim]Cl, 0.1 mol of 1-propyl-3-methylimidazolium bisulfate [pmim] HSO 4 0.1mol, reaction temperature 60~100℃, reaction 120min, after the reaction is completed, add a certain amount of water, extract the reaction solution with cyclohexane, wash and dry the organic phase with water, spin off the solvent to obtain 1-chlorooctane, the product yield The rate is 82%. Add 0.2mol of sodium chloride to the water phase, and the others are the same as in Example 1.
Embodiment 3
[0061] In the reaction system, add 1,7-heptanediol 0.2mol, 1-isopropyl 3-methylimidazolium iodide [ipmim]I 0.4mol, 1-isopropyl-3-methylimidazolium bisulfate [ipmim] ] HSO 4 0.4mol, nitrogen protection, reaction temperature 60 ~ 100 ℃, reaction 30min, after the reaction, add a certain amount of water, extract the reaction solution with cyclohexane, wash and dry the organic phase, spin off the solvent to obtain 1,7 -Diiodoheptane, the product yield is 90%. Add 0.5mol of sodium iodide to the aqueous phase, and the others are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com