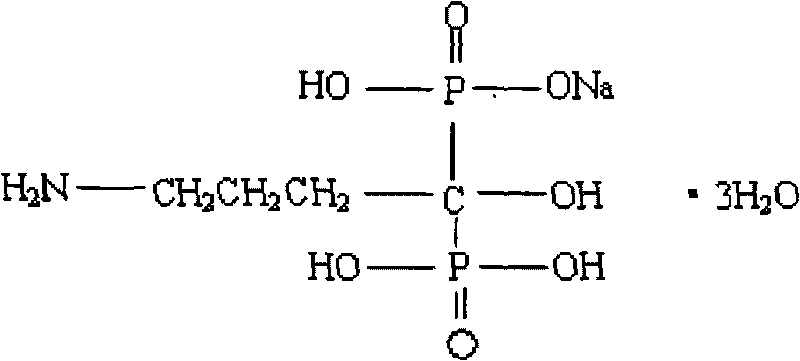
Alendronate sodium enteric-coated tablet and preparation method thereof
A technology of alendronate sodium and enteric-coated tablets, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, and drug delivery, etc., can solve the problems of expensive calcitonin, inability to restore bone tissue, and side effects, and achieve The effect of rapid drug dissolution, convenient administration and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Tablet prescription:
[0026] Alendronate Sodium 10g
[0027] Lactose 45g
[0028] Microcrystalline Cellulose 40g
[0029] Micronized silica gel 2.5g
[0030] Magnesium Stearate 2.5g
[0031] 30% ethanol solution appropriate amount
[0032]
[0033] 1000 pieces
[0034] Coating Solution Prescription
[0035] Hypromellose 5g
[0036] Acrylic resin 2.5g
[0037] 30% ethanol solution appropriate amount
[0038] Tween-80 5g
[0040]
[0041] 100ml
[0042] The alendronate sodium, microcrystalline cellulose, lactose, micropowder silica gel that takes by weighing recipe quantity crosses 24 mesh sieves and makes it mix homogeneously. Mix in 30% ethanol solution, then add the solution into the mixed fine powder of raw and auxiliary materials, and stir to make a soft material. Pass the soft material through a 20-mesh sieve to make granules. Dry at 70°C for 4 hours, pass throu...
Embodiment 2
[0044] Tablet prescription:
[0045] Alendronate Sodium 10g
[0046] 35g pregelatinized starch
[0047] Microcrystalline Cellulose 40g
[0048] Sodium carboxymethyl starch 13g
[0049] Micronized silica gel 0.5g
[0050] Magnesium Stearate 1.5g
[0051] 30% ethanol solution appropriate amount
[0052]
[0053] 1000 pieces
[0054] Coating Solution Prescription
[0055] Hypromellose 2.5g
[0056] Acrylic resin 2.5g
[0057] 30% ethanol solution appropriate amount
[0058] Tween-80 2.5g
[0060]
[0061] 100ml
[0062]The alendronate sodium, pregelatinized starch, microcrystalline cellulose, carboxymethyl starch sodium of prescription quantity are taken by weighing 24 mesh sieves and make it mix homogeneously. Add 30% ethanol solution and mix well, stir to make soft material. Pass the soft material through a 20-mesh sieve to make granules. Dry at 70°C for 4 hours, pass through a 2...
Embodiment 3
[0063] Embodiment 3: tablet core prescription
[0064] Alendronate Sodium 10g
[0065] 30g pregelatinized starch
[0066] Microcrystalline Cellulose 40g
[0067] Sodium carboxymethyl starch 15g
[0068] Micronized silica gel 2.5g
[0069] Magnesium Stearate 2.5g
[0070] 30% ethanol solution appropriate amount
[0071]
[0072] 1000 pieces
[0073] Coating Solution Prescription
[0074] Hypromellose 2g
[0075] Acrylic resin 1.5g
[0076] 30% ethanol solution appropriate amount
[0077] Tween-80 1.25g
[0079]
[0080] 100ml
[0081] The alendronate sodium, pregelatinized starch, microcrystalline cellulose, carboxymethyl starch sodium of prescription quantity are taken by weighing 24 mesh sieves and make it mix homogeneously. Add the prescribed amount of sodium chloride into 30% ethanol solution of 8% povidone and mix evenly, then add the solution into the mixed fine powder o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com