Nanometer europium fluorescent particle with performance of visible light excitation, preparation method and application thereof
A technology of fluorescent particles and visible light, which is applied in the field of preparation of nano-europium fluorescent particles, can solve the problems of easy leakage of fluorescent molecules, complicated labeling process, background fluorescence interference, etc., to prolong continuous measurement time, improve photobleaching resistance, and improve fluorescence Effect of Luminous Intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
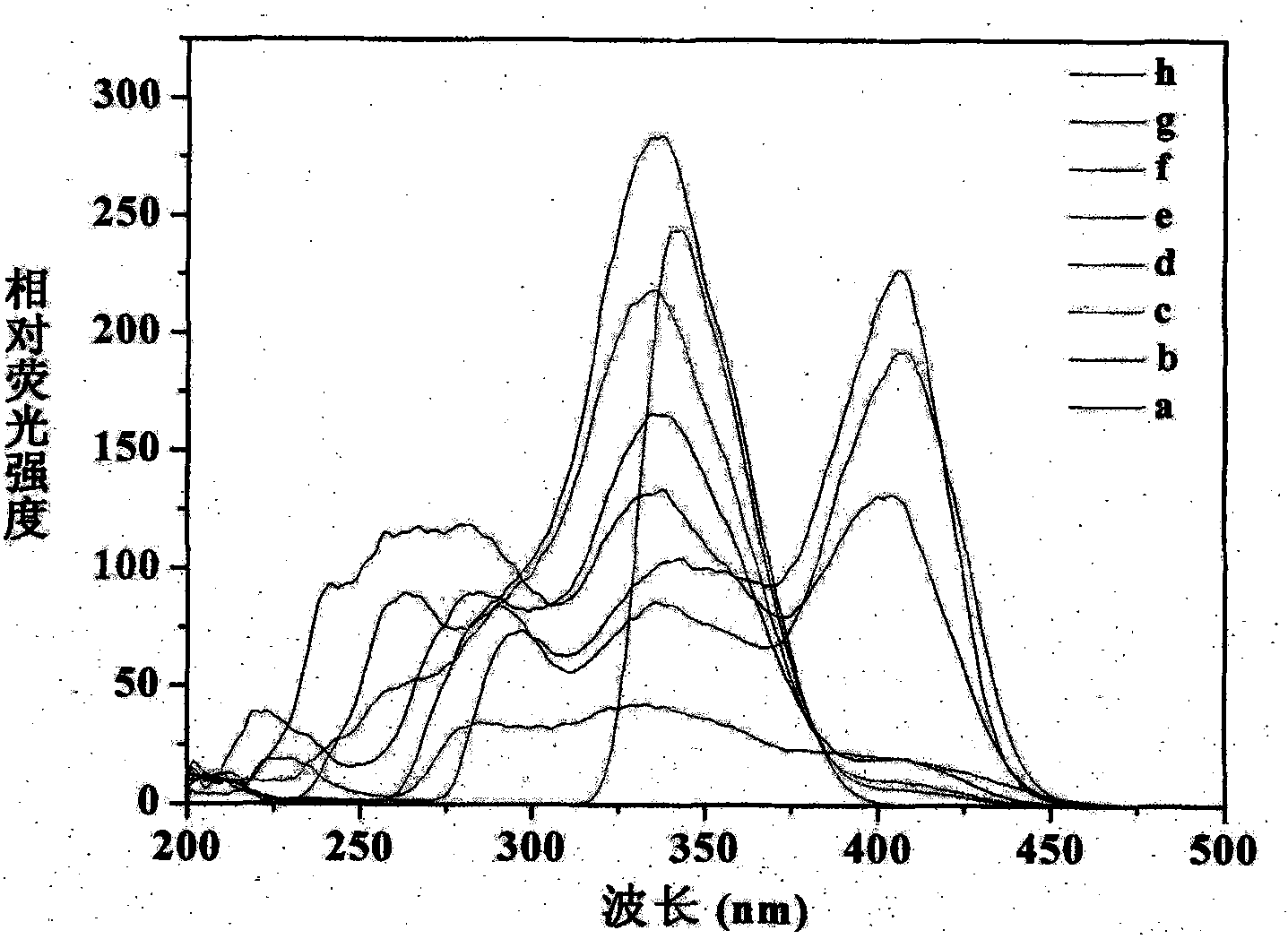
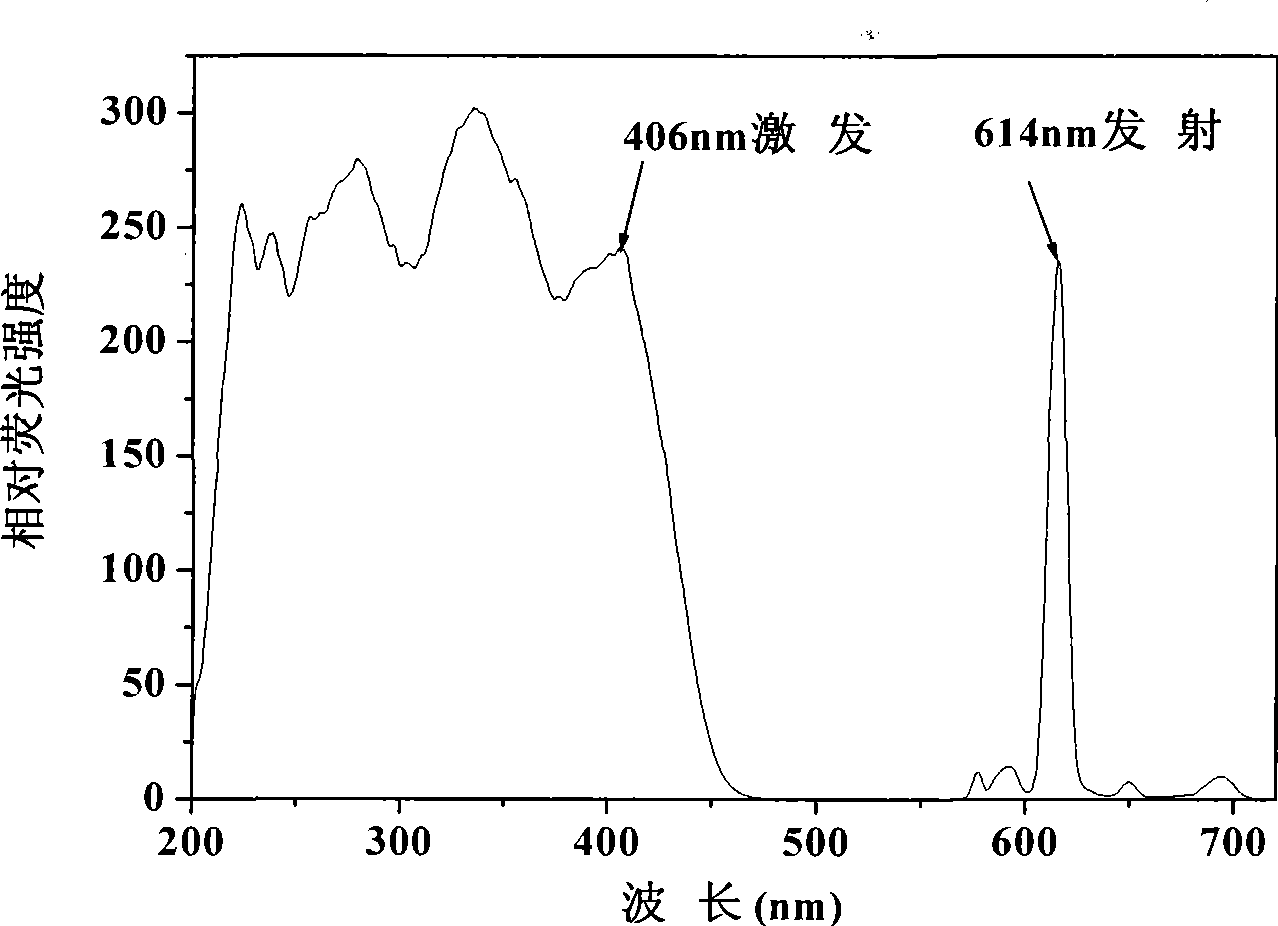
[0038] Example 1: Preparation and Characterization of Nano Europium Fluorescent Particles Excited by Visible Light
[0039] (1) Fluorescent precursor APS-BHHCT-Eu 3+ - Preparation of DPBT
[0040] Dissolve 25 μmol of BHHCT in 4 mL of anhydrous tetrahydrofuran, add 62.5 μmol of aminopropyltriethoxysilane (APS) under stirring, stir at room temperature for 3 hours, then add 15 mL of anhydrous tetrahydrofuran solution dissolved in 50 μmol of DPBT, and add 25 μmol EuCl 3 ·6H 2 O, stirred for 24 hours, evaporated the solvent to dryness with a rotary evaporator and dried in vacuum to obtain the fluorescent precursor APS-BHHCT-Eu 3+ -DPBT.
[0041] (2) Preparation of nano-europium fluorescent bioprobes excited by visible light
[0042] The above fluorescent precursor APS-BHHCT-Eu 3+ - DPBT (2 mg, 4 mg, 6 mg, 8 mg and 10 mg) was dissolved in 1.5 mL of toluene and added to a solution containing 7.25 g of cyclohexane, 2.37 g of Triton X-100, 1.82 g of n-octanol and 0.55 mL of water...
Embodiment 2
[0053] Example 2: Nano europium fluorescent particles are used as markers for time-resolved fluorescent immunoassay for human prostate-specific antigen (PSA for short)
[0054] (1) Nano europium fluorescent particles labeled streptavidin (SA for short)
[0055] Add 1.0 mg of nano-europium fluorescent particles and 3.0 mg of bovine serum albumin (BSA) with 0.1 mol L of pH 7.1 -1 After 1.2 mL of phosphate buffer solution was completely dissolved, 0.3 mL of 1% glutaraldehyde aqueous solution was added under stirring, and stirred at room temperature for 24 hours. After the nanoparticles were collected by centrifugation and fully washed with phosphate buffer solution, 0.9 mL of phosphate buffer solution containing 0.2 mg of SA and 0.2 mL of 1% glutaraldehyde aqueous solution were added, stirred at 4 °C for 22 hours, and 2.0 mg of sodium borohydride was added to continue the reaction 2 Hour. The nanoparticles marked with SA molecules on the surface were centrifuged and fully washe...
Embodiment 3
[0062] Example 3: Nano-europium fluorescent particles are used as markers for time-resolved fluorescence microscopy imaging to determine pathogenic microorganisms in complex environmental samples-Giardia lamblia (Giardia lamblia)
[0063] (1) Time-resolved fluorescence microscopy imaging of Giardia lamblia in complex environmental samples Take 5 μL of Giardia lamblia (2×10 6 1 / mL) was added to 15 μL of 10000-fold concentrated environmental water samples containing a large number of various impurities, and then 16 μL of anti-Giardia lamblia monoclonal antibody (50 μg / mL), 16 μL of biotin-labeled rabbit anti-mouse II Secondary antibody (50 μg / mL, prepared in the same way as biotin-labeled goat anti-human PSA antibody) and 6 μL nanofluorescent particle-labeled SA solution, incubated at room temperature for 24 hours, low-speed centrifugation (500 rpm) to collect Giardia lamblia and Redisperse in water several times to remove unreacted nanoparticles and antibodies. The Giardia lam...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com