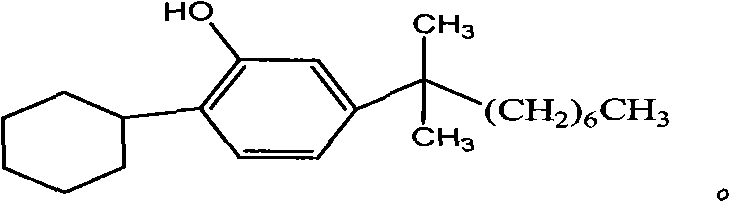
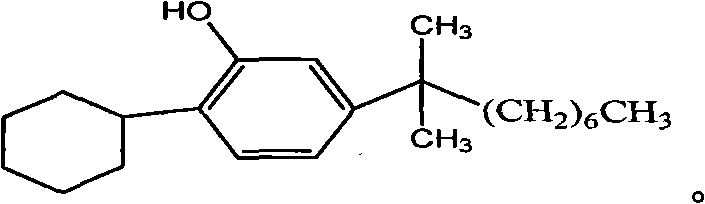
2-cyclohexyl-5-(1,1- dimethyl octyl) phenol and synthesizing method thereof
A technology of dimethyloctyl and synthesis methods, which is applied in the directions of active ingredients of hydroxyl compounds, chemical instruments and methods, drug combinations, etc., to achieve the effects of high yield, short synthesis route and easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
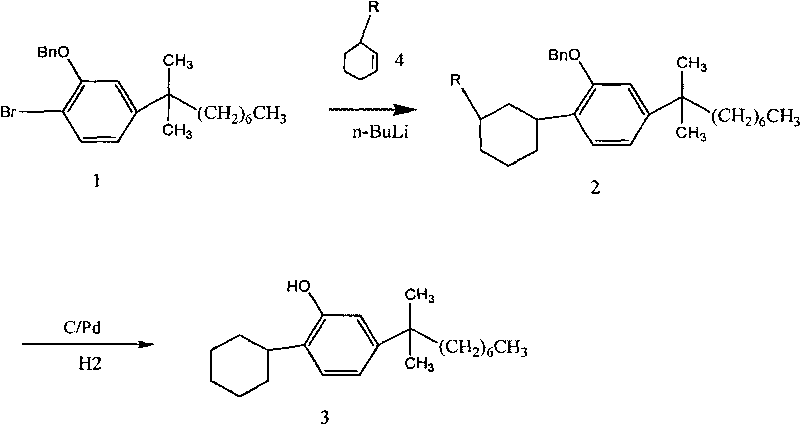
[0017] Example 1: Synthesis of 2-benzyloxy-1-cyclohexyl-4-(1,1-dimethyloctyl)benzene
[0018] In a well-dried 500ml reaction flask, 60g 2-benzyloxy-1-bromo-4-(1,1-dimethyloctyl)benzene was dissolved in 300ml dichloromethane. Under the protection of argon gas, Add 65g of n-butyllithium dropwise within 30 minutes at -25°C, increase the temperature in the reaction to about 30°C after the addition is complete, react at this temperature for 30 minutes, cool to -20°C, and then add 20g dropwise to the reactor The mixed solution of cyclohexene and 20g of dichloromethane, continue to stir the reaction for 40min, after the reaction is over, add 60ml of 1N dilute hydrochloric acid, wash the organic phase twice with water, and then use saturated NaHCO 3 Wash with aqueous solution and collect the organic phase. The solvent was removed from the organic phase by rotary evaporation, and 48 g of pale yellow oil was obtained after evaporation to dryness.
Embodiment 2
[0019] Example 2: Synthesis of 2-benzyloxy-1-(3'-methoxycyclohexyl)-4-(1,1-dimethyloctyl)benzene
[0020] In a well-dried 500ml reaction flask, dissolve 60g 2-benzyloxy-1-bromo-4-(1,1-dimethyloctyl)benzene in 300ml ether. Under the protection of argon, 100g of n-butyllithium was added dropwise within 30 minutes at ℃. After the addition, the temperature in the reaction was increased to about 30°C, reacted at this temperature for 45 minutes, cooled to -25°C, and then 25g methoxy was added dropwise to the reactor The mixed solution of cyclohexene and 25g ether, continue to stir the reaction for 60min, after the reaction is over, add 90ml of 1N dilute hydrochloric acid, wash the organic phase twice with water, and then use saturated NaHCO 3 Wash with aqueous solution and collect the organic phase. The solvent was removed from the organic phase by rotary evaporation, and 51 g of light yellow oil was obtained after evaporation to dryness.
Embodiment 3
[0021] Example 3: Synthesis of 2-benzyloxy-1-(3'-cyclohexyloxycyclohexyl)-4-(1,1-dimethyloctyl)benzene
[0022] In a well-dried 500ml reaction flask, 60g 2-benzyloxy-1-bromo-4-(1,1-dimethyloctyl)benzene was dissolved in 300ml dichloromethane. Under the protection of nitrogen gas, Add 80g n-butyllithium dropwise within 30 minutes at 10°C. After the dropwise addition, increase the internal temperature of the reaction to about 30°C, react at this temperature for 50 minutes, cool to -30°C, and then drop 110g ring into the reactor. The mixed solution of hexyloxycyclohexene and 110g of dichloromethane, continue to stir the reaction for 60min, after the reaction is over, add 60ml of dilute hydrochloric acid, wash the organic phase twice with water, and then use saturated NaHCO 3 Wash with aqueous solution and collect the organic phase. The solvent was removed from the organic phase by rotary evaporation, and 60 g of light yellow oil was obtained after evaporation to dryness.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com