Bimetal methanation catalyst and preparation method thereof
A methanation catalyst, catalyst technology, applied in catalyst activation/preparation, metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, etc., can solve problems such as removing hydrogen-rich gas, and achieve high and low temperature Methanation activity, easy reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
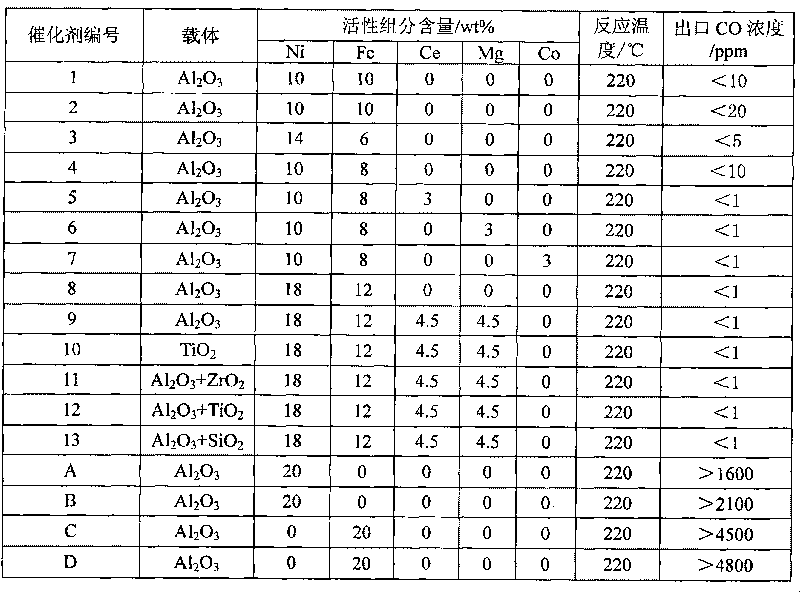
[0026] Take 100g of alumina carrier and treat it at 150°C and 13.3Pa pressure for 10min, then cool it down to room temperature for later use; weigh 49.4g of nickel nitrate hexahydrate and 72.4g of iron nitrate nonahydrate, and prepare 200mL of mixed salt solution; take 200mL of prepared The mixed salt solution was impregnated into 100g of the treated carrier, and stood at room temperature for 2h; then filtered off the excess solution, dried at 120°C for 6h, and was heated to 450°C for 5h to decompose the salt. 2 Reduction treatment was carried out at 400° C. for 10 h in a stream, and after cooling down to room temperature, it was passivated by oxygen to obtain catalyst 1.
Embodiment 2
[0028] Take 100g of alumina carrier and treat it at 150°C and 13.3Pa pressure for 10min, then cool it down to room temperature for later use; weigh 24.7g of nickel nitrate hexahydrate, 21.2g of nickel acetate tetrahydrate, 36.2g of ferric nitrate nonahydrate, 22.1g of acetic acid tetrahydrate Iron, prepared into 200mL mixed nickel salt solution; take 200mL of the prepared mixed nickel salt solution and impregnate it into 100g of the treated carrier, let stand at room temperature for 2h; filter off excess solution, dry at 120°C for 6h, heat up to 450°C for roasting 5h to decompose the salt, then in H 2 Reduction treatment was carried out at 400° C. for 10 h in a stream, and after cooling down to room temperature, the catalyst 2 was passivated by oxygen.
Embodiment 3
[0030] Take 100g of alumina carrier and treat it at 150°C and 13.3Pa pressure for 10min, then cool it down to room temperature for later use; weigh 69.4g of nickel nitrate hexahydrate and 43.4g of iron nitrate nonahydrate, and prepare 200mL of mixed nickel salt solution; take 200mL of prepared Immerse the good mixed nickel salt solution into 100g of the treated carrier, and let it stand at room temperature for 2h; then filter off the excess solution, dry at 120°C for 6h, heat up to 450°C and roast for 5h to decompose the salt, and then in 50% H 2 / 50%N 2 Reduction treatment was carried out at 400° C. for 10 h in the stream, and after cooling down to room temperature, the catalyst 3 was passivated by oxygen.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com