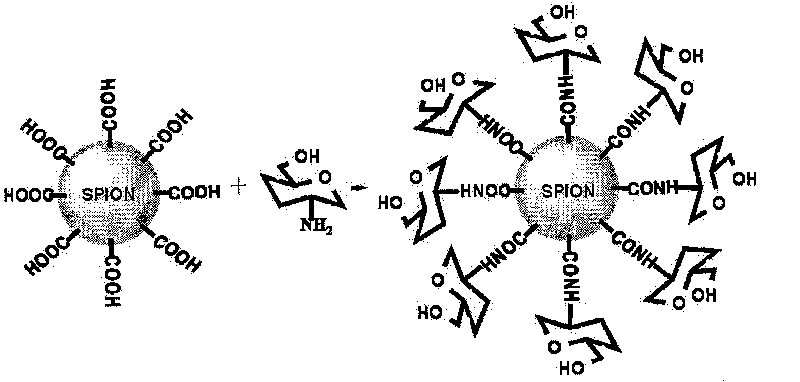
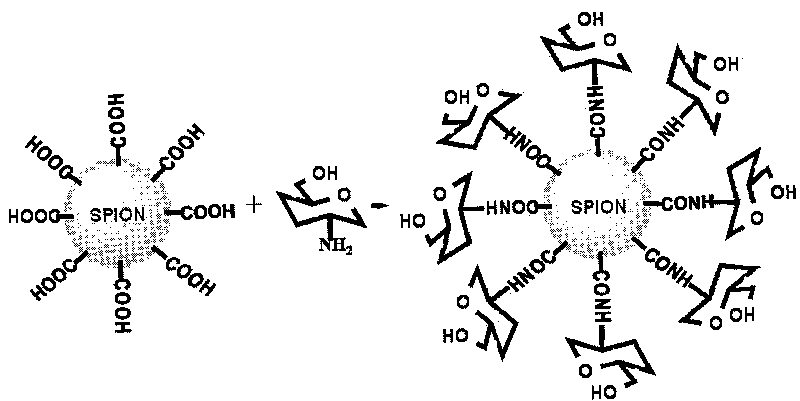
D-glucosamine-modified iron oxide nanoparticles and preparation method of lyophilized powder thereof
A technology of iron oxide nanoparticles and glucosamine, which is applied in the fields of emulsion delivery, MRI/MRI contrast agent, drug delivery, etc. It can solve the problems of not targeting tumors and affecting the application effect in the field of tumor targeting diagnosis, etc. Achieve a non-irritating effect on blood vessels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Add 0.1 mol of carbodiimide to 0.1 mol of citric acid-modified superparamagnetic iron oxide nanoparticles colloidal solution, mix well, and activate the carboxyl groups on the surface of iron oxide nanoparticles for 30 minutes at room temperature; add 0.1 mol of D-Glucosamine, mixed well, reacted with shaking at room temperature for 12 hours; dialyzed the above system with 50 times double distilled water for 10 hours; added 3% sorbitol to the system, heat sterilized, pre-frozen at -20°C for 12 hours, and freeze-dried for 10 hours. Hours, that's it.
Embodiment 2
[0021] Example 2: Add 0.5 mol carbodiimide and 0.1 mol N-hydroxysuccinimide to 0.1 mol dimercaptosuccinic acid-modified superparamagnetic iron oxide nanoparticle colloidal solution, mix well, and activate iron oxide nanoparticles at room temperature Carboxyl groups on the surface of grains for 60 minutes; add 0.2mol D-glucosamine to the above system, mix well, and react with shaking at room temperature for 24 hours; dialyze the above system with 100 times double distilled water for 12 hours; add 5% mannitol and 1% polyethylene glycol to the system Diol, sterilized by heating at 100°C for 30 minutes; pre-freezing at -40°C for 12 hours, then freeze-drying for 24 hours to obtain the product.
Embodiment 3
[0022] Example 3: Add 0.3 mol carbodiimide and 0.08 mol N-hydroxysuccinimide to 0.1 mol carboxy-terminal polyethylene glycol derivative modified superparamagnetic iron oxide nanoparticle colloidal solution, mix well, and activate at room temperature Carboxyl groups on the surface of iron oxide nanoparticles for 45 minutes; add 0.1mol D-glucosamine to the above system, mix well, and react with shaking at room temperature for 15 hours; dialyze the above system with 100 times double distilled water for 12 hours; add 5% glucose to the system, Sterilize with a 0.22 μm filter membrane; pre-freeze at -30°C for 10 hours, and then freeze-dry for 18 hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com