Method for splitting DL-p-hydroxymandelic acid
A technology of p-hydroxymandelic acid and a resolving agent is applied in the field of preparing high-purity D-p-hydroxymandelic acid, and can solve the problem that the splitting of DL-p-hydroxymandelic acid has little reference value, low product purity, and many operation steps. and other problems, to achieve the effect of high-efficiency splitting efficiency, low-cost solvent, and improved reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
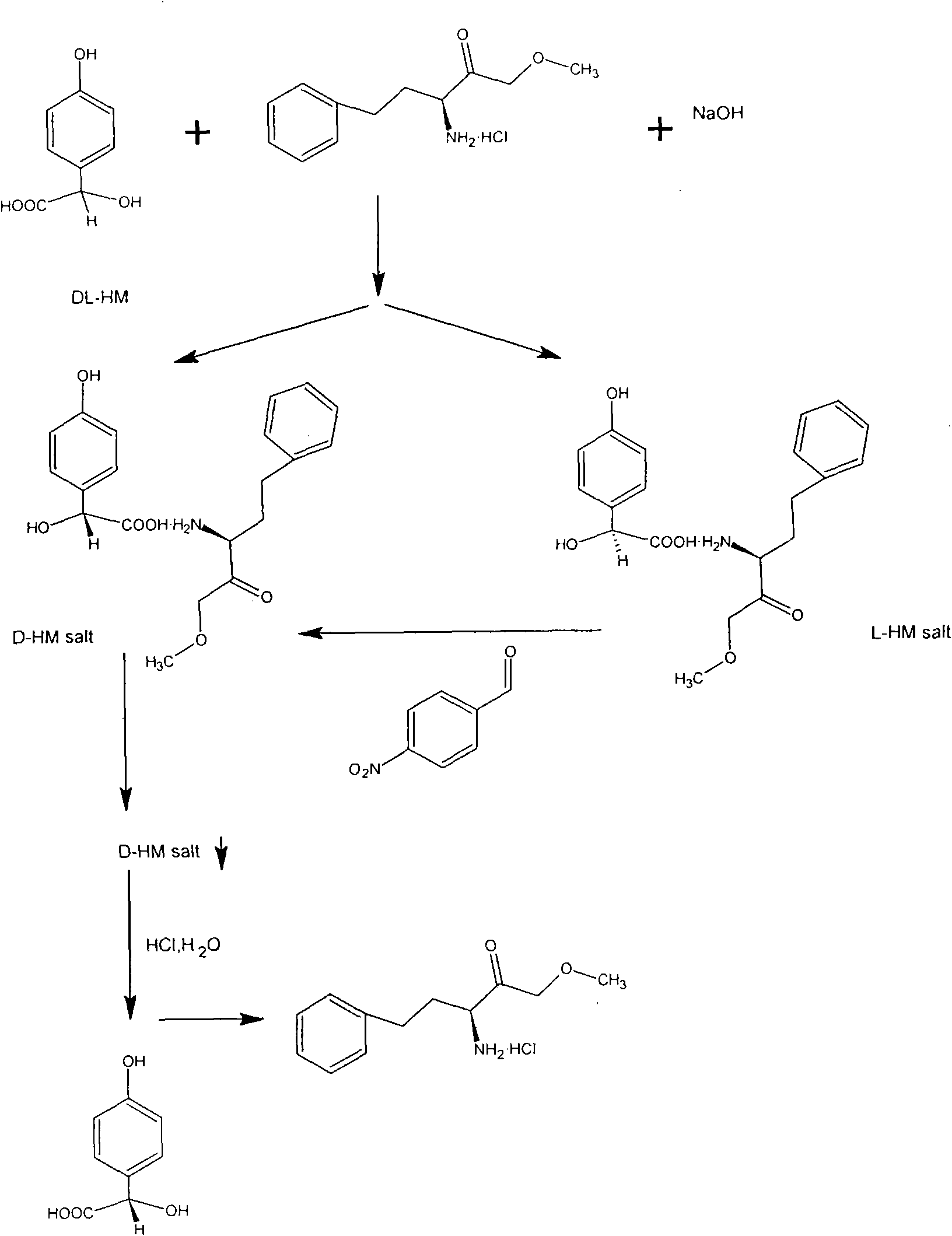
[0050] Get DL-p-hydroxymandelic acid 8.4 grams (0.05mol), (S)-(+)-2-amino-4-phenylbutyric acid ethyl ester hydrochloride 12.18 grams (0.05mol), drop into 120ml 30% In ethanol aqueous solution, start stirring, N 2 Protect, heat up to 75°C to form a transparent reaction solution, then slowly add 2 grams (0.05mol) of sodium hydroxide, continue to stir the reaction for 30min, a white precipitate occurs, slowly cool down to 10°C, let the formed D- The double salt of p-hydroxymandelic acid is fully separated. Filtration under reduced pressure obtains filtrate A and 9.07 grams of white crystals, and yield is 97% of theoretical yield, and this white crystals is D-parahydroxymandelic acid double salt (D-parahydroxymandelic acid (S)-(+) -2-amino-4-phenylbutyric acid ethyl ester), washed with a small amount of ethanol, and dried in a vacuum oven.
[0051] Take by weighing 9.07 grams of dried D-p-hydroxymandelic acid (S)-(+)-2-amino-4-phenylbutyric acid ethyl ester 9.07 grams, be dissol...
Embodiment 2
[0055] Get (S)-(+)-2-amino-4-phenylbutyric acid ethyl ester hydrochloride 12.18 grams (0.05mol), drop into 120ml mass percent concentration and be 50% ethanol aqueous solution, start stirring, N 2 protection, the temperature was raised to 55° C., and then 2 g (0.05 mol) of sodium hydroxide was slowly added to form a transparent reaction solution. Then add 8.4 g (0.05 mol) of DL-p-hydroxymandelic acid, continue to stir the reaction for 80 minutes, a white precipitate occurs, slowly cool down to 10°C, and allow the formed D-p-hydroxymandelic acid double salt to be fully separated. 9.18 grams of white crystals obtained by filtration under reduced pressure and filtrate A, the yield is 98.2% of the theoretical yield, the white crystals are D-p-hydroxymandelic acid (S)-(+)-2-amino-4-benzene ethyl butyrate, washed with a small amount of ethanol, and dried in a vacuum oven.
[0056] Take by weighing dried D-hydroxymandelic acid · (S)-(+)-2-amino-4-phenylbutyric acid ethyl ester 9.18 ...
Embodiment 3
[0060] 12.2 grams of recovered (S)-(+)-2-amino-4-phenylbutyric acid ethyl ester hydrochloride and 8.4 grams of DL-p-hydroxymandelic acid were weighed and mixed, and other conditions were the same as in Example 1. Finally, 3.75 grams of D-p-hydroxymandelic acid was obtained, with a theoretical yield of 93.3%. D-hydroxymandelic acid has been tested, ; Chemical purity greater than 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com