Uloric crystal and preparation method thereof
A technology of febuxostat and crystal form, which is applied in the field of α crystal form of febuxostat and its preparation, can solve the problem of different solubility, melting point, and dissolution bioavailability, which affect drug stability, bioavailability and curative effect and other problems, to achieve the effect of stable crystal shape, good solubility and simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Preparation of febuxostat α crystal form:
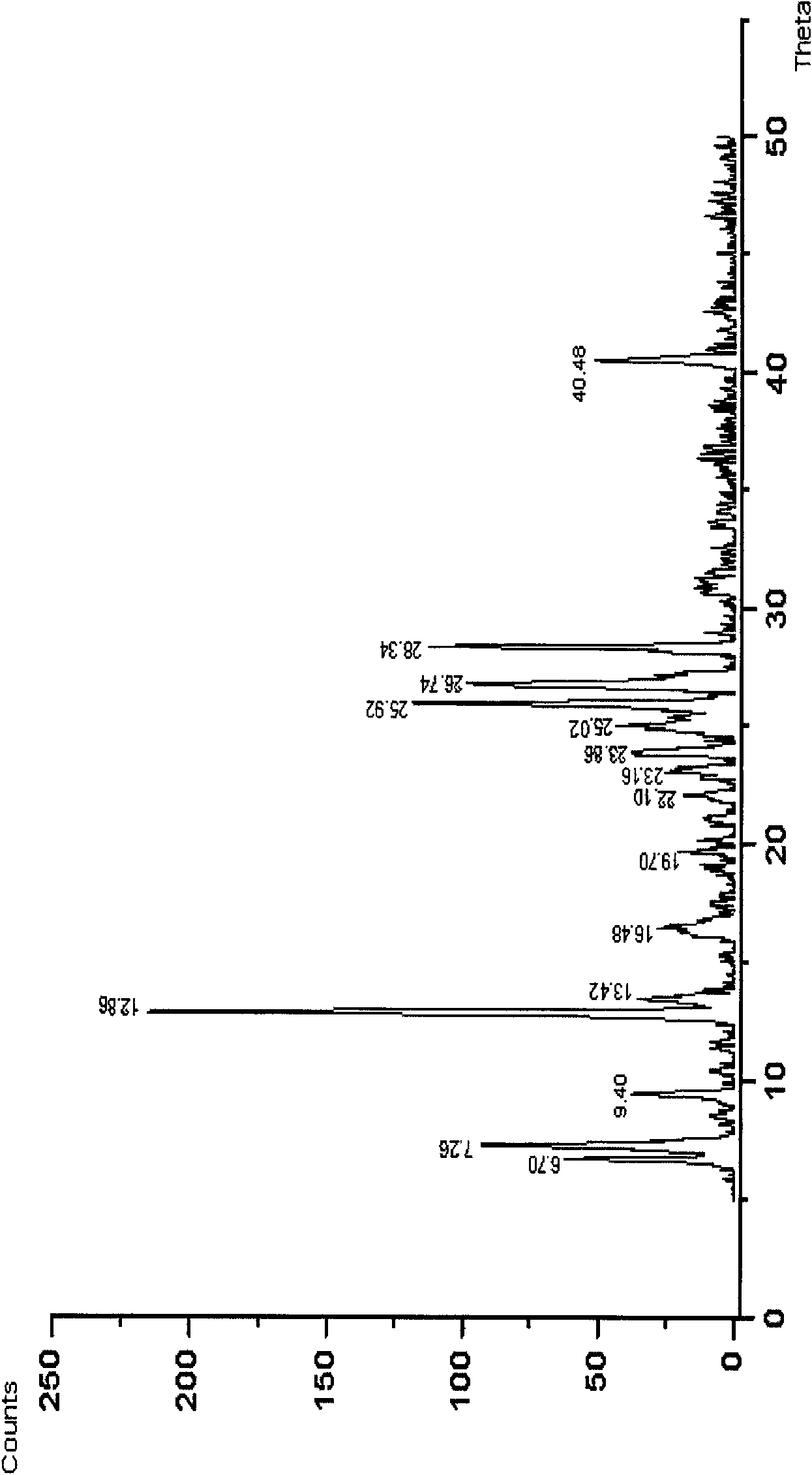
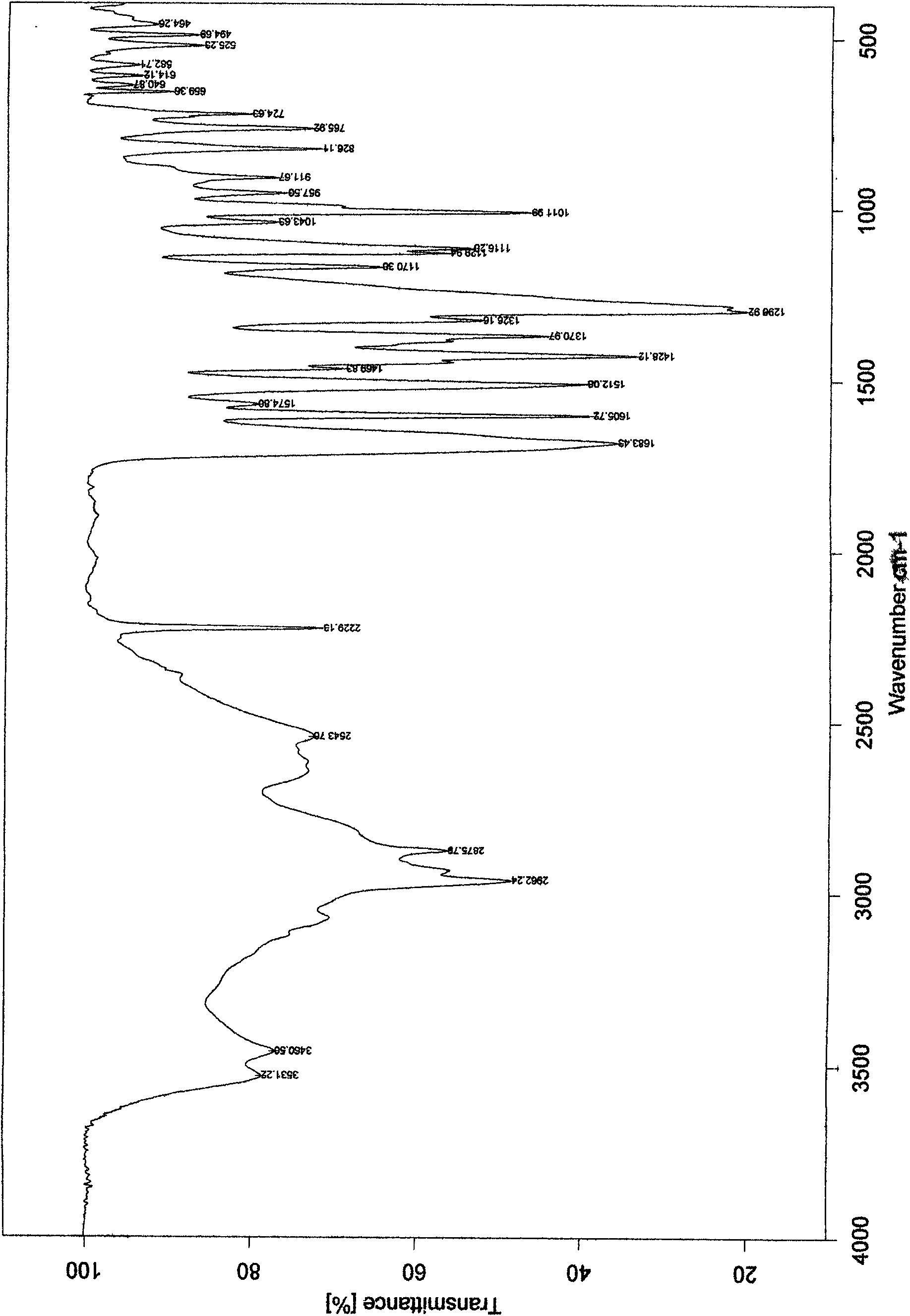
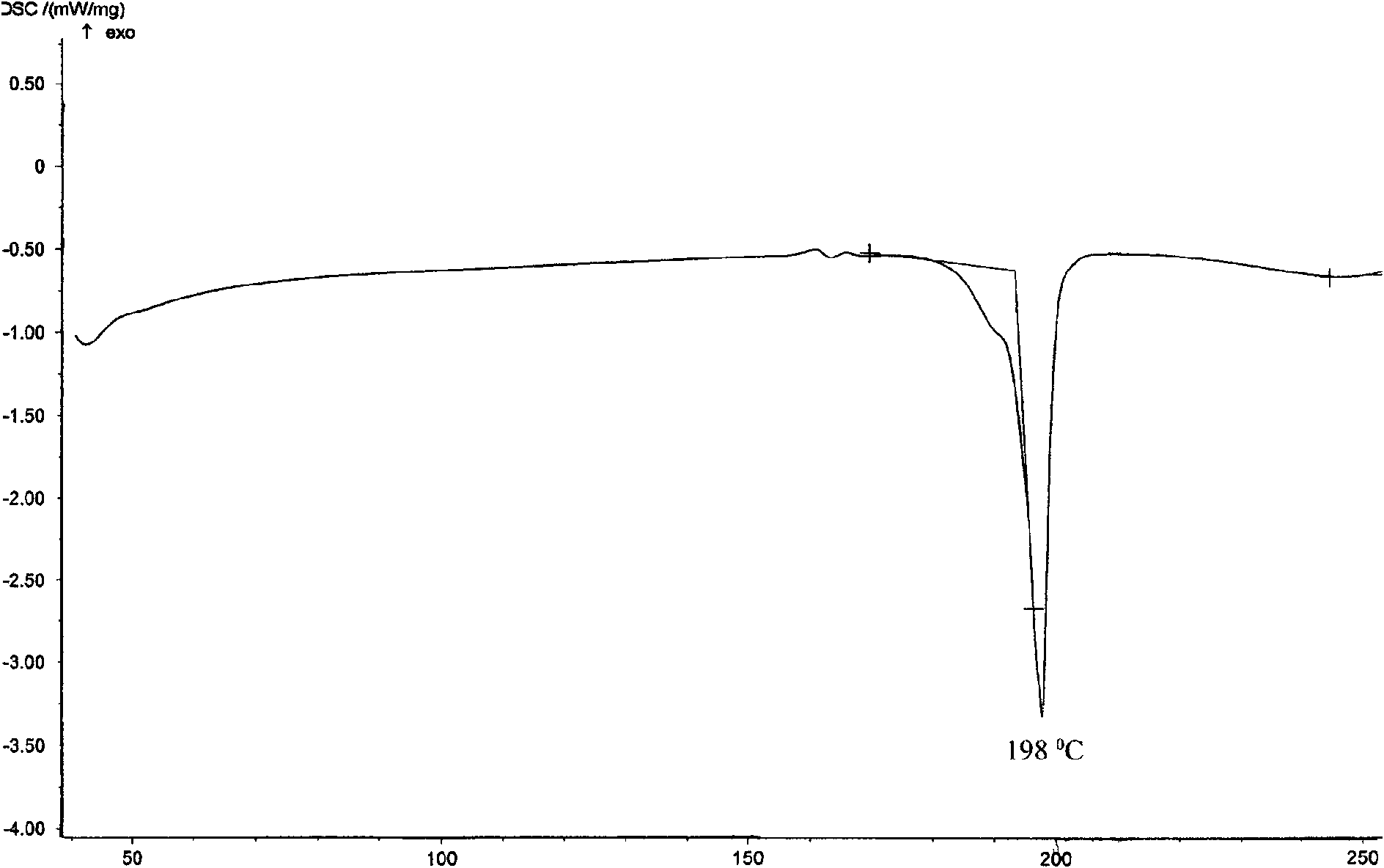
[0021] Put 1 g of febuxostat in a 100 ml single-necked flask, add 70 ml of ethylene glycol monomethyl ether, heat and stir in an oil bath at 60°C until completely dissolved. Extract the solvent under reduced pressure at 60°C for crystallization, wash with water, filter, and dry under reduced pressure at 60°C for 8 hours to obtain crystalline powder. Determination of its powder diffraction pattern see figure 1 , see the infrared spectrum figure 2 , according to the powder diffraction pattern and infrared spectrum, it is obvious that the crystalline form α is generated. Yield: 90%, mp: 198-200°C.
Embodiment 2
[0023] Stability test of febuxostat α crystal form
[0024] The α crystal of febuxostat prepared in Example 1 was respectively placed under the conditions of 4500LX, 60°C and RH92.5%, and samples were taken on day 0, day 5, and day 10 for content determination and relevant room determination, and X was drawn - powder diffraction pattern and infrared absorption spectrogram, the results are shown in Table 1.
[0025] The condition and the method of content determination are: high performance liquid chromatography, with methanol-0.01mol / L potassium dihydrogen phosphate solution (75:25) adjust pH value to 5.4 ± 0.1 with 5% phosphoric acid as mobile phase; Detection wavelength λ= 315nm; the preparation of the test solution and the reference solution: accurately weigh about 30mg of the fine powder of the sample, put it in a 100ml measuring bottle, add mobile phase to ultrasonic to dissolve, add mobile phase to the scale, shake well, accurately measure 10ml, put In a 100ml measuring...
Embodiment 3
[0031] Solubility test of febuxostat α crystal form and A crystal form
[0032] The febuxostat α crystal and A crystal were fully ground respectively, and prepared saturated solutions with water, 0.1N HCl, PH5.0 phosphate buffer solution and PH6.8 phosphate buffer solution (ultrasound for 30 minutes, there was still a white solid in the solution substance exists), the content of febuxostat in the saturated solution was determined by ultraviolet spectrophotometry, and the results are shown in Table 2. It can be seen from Table 2 that the solubility of the α crystal form is about double that of the A crystal form.
[0033] Table 2 α crystal form and A crystal form solubility test results
[0034]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com