Livestock peptide vaccine and preparation method thereof
A peptide vaccine and vaccine technology, which is applied in the field of veterinary foot-and-mouth disease chemical synthesis peptide vaccine and its preparation, can solve the problems of limited application, and achieve the effects of no biological safety, good application prospects, and easy large-scale synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0036] Example I. Solid Phase Chemical Synthesis of Peptide Antigens
[0037] The preparation of the peptide antigen of the present invention can use the classic solid-phase chemical synthesis method using a fully automatic polypeptide synthesizer, using FMOC to protect amino acids, and using amino resin as the solid-phase carrier. The production process is usually divided into solid-phase synthesis of peptide antigens, cleavage of peptides, purification of antigens, and sterilized storage. Specific steps are as follows:
[0038] 1.1 Solid-phase synthesis of peptide antigens
[0039] 1.1.1 Preparation of synthetic raw materials
[0040]The sequences of the synthetic peptide antigens are shown as: SEQ ID NO:1, SEQ ID NO:2, SEQ ID NO:3, SEQ ID NO:4.
[0041] Prepare suitable protected amino acids according to the above antigen sequence and synthesis scale, and add them to corresponding amino acid bottles. Also weigh the amino resin as required, put it into the reaction chamb...
Embodiment II
[0069] Example II. Proliferation assay of exogenous T cell epitopes on T cells
[0070] Test purposes:
[0071] The inventor found a powerful exogenous T cell epitope (SEQID NO: 4) on Peste des petits ruminants virus that can enhance the dominant B cell epitope at positions 130-168 on FMD VP1 through a large number of experiments, and then passed a certain The linker amino acid connects the potent exogenous T cell epitope and the dominant B cell epitope, and constructs a peptide antigen containing a helper potent exogenous T cell epitope and the main epitope gene of FMDV, which is compatible with the 130th- The antigen composed of the dominant B cell epitope at position 168 was subjected to a T cell proliferation test to examine the immunostimulatory effect of the exogenous epitope SEQ ID NO: 4 on T cells.
[0072] Test preparation:
[0073] 96-well plate, incubator, Bio-Rad microplate reader, biological safety cabinet
[0074] Preparation of 3-(4,5-dimethylthiazole-2)-2,5-...
Embodiment III
[0082] The safety test of embodiment III peptide vaccine
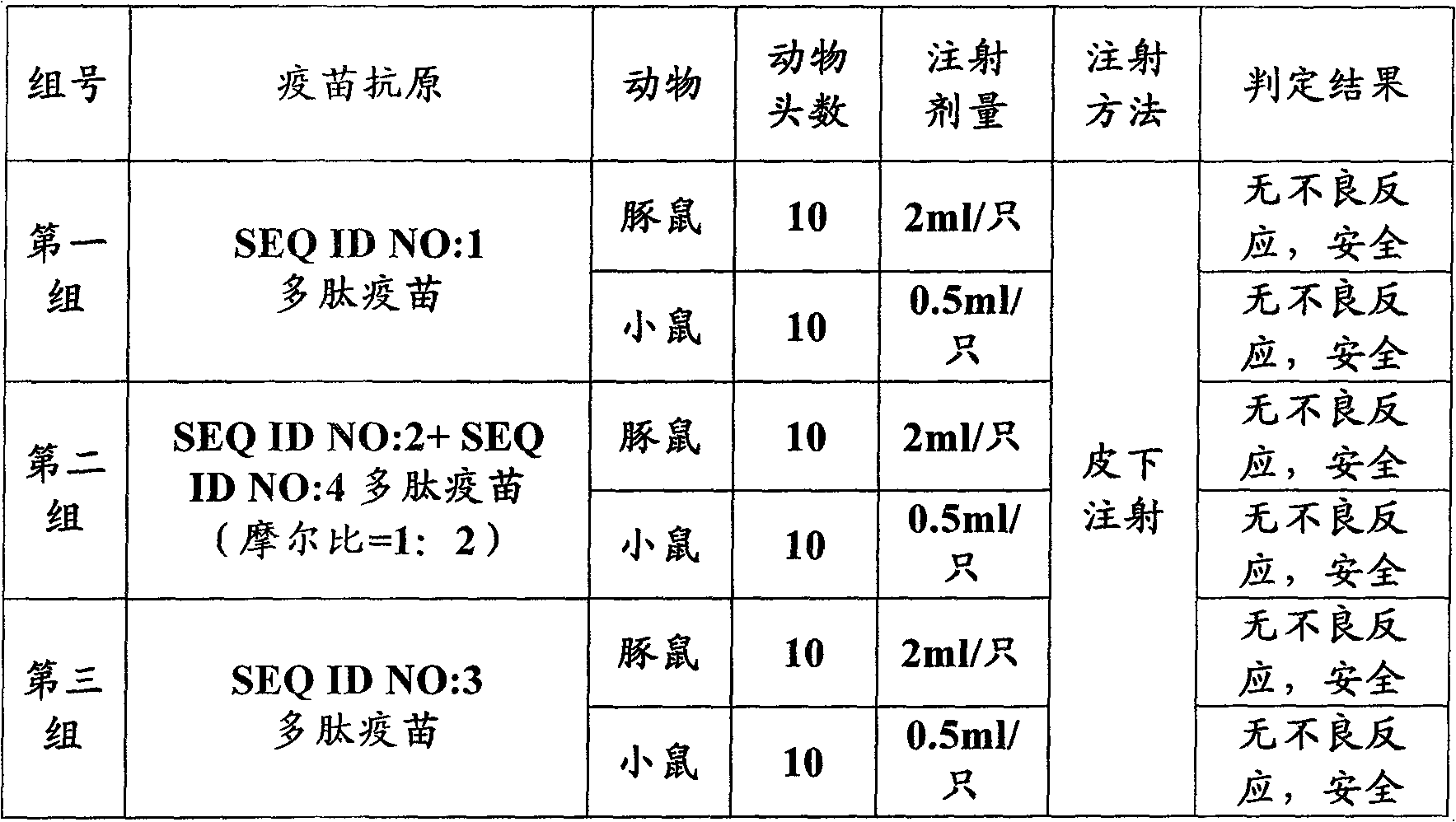
[0083] According to above-mentioned embodiment synthesis as the polypeptide antigen of aminoacid sequence shown in SEQ ID NO: 1, SEQ ID NO: 2, SEQ ID NO: 3, SEQ ID NO: 4, then prepare the polypeptide vaccine of anti-foot-and-mouth disease virus according to above-mentioned embodiment, every The antigen content of the batch vaccine is 60ug / ml. For each batch of vaccine, 10 guinea pigs with a body weight of 350-450 g were used for subcutaneous injection of 2 ml each; 10 mice with a body weight of 18-22 g were used for each subcutaneous injection of 0.5 ml. Continuously observe for 7 days to determine whether there is death or obvious local adverse reaction or systemic reaction caused by the injection. The observation results are shown in Table 2.
[0084] Table 2: Safety test results of peptide vaccines on guinea pigs and mice
[0085]
[0086] It can be seen from the results of this test that no death or obvious l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com