Foot-and-mouth disease O-type antigen epitope polypeptide and its preparation method and application
An antigen peptide, foot-and-mouth disease technology, applied in the field of medicine, can solve the problems affecting the use of new vaccines, immune effects, economic problems, and inability to effectively protect animals, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1, the solid-phase synthesis of polypeptide antigen of foot-and-mouth disease synthetic peptide vaccine
[0025] The present invention studies the variation of the main antigenic sites of the FMD by determining the sequence of the newly popular strains of the domestic FMD and combining the sequences of the FMD vaccine strains, and counts the variation frequency of the main mutated amino acid sites, and at the same time combines computer-aided FMD Analysis and prediction of antigenic sites, chemical synthesis of possible antigenic site peptides, that is, according to the statistical variation frequency of variable sites, different amino acids are used at these sites to obtain a multiplicity covering all current possible variable sites A candidate polypeptide antigen. Furthermore, these candidate polypeptide antigens were screened through a large number of animal experiments, and the polypeptide antigens capable of inducing an immune response in animals with a...
Embodiment 2
[0057] Embodiment 2, the preparation of foot-and-mouth disease O type synthetic peptide vaccine
[0058] 1.1 Preparation of antigen aqueous phase
[0059] Weigh the synthetic peptide antigens represented by polypeptide 1 or polypeptide 2 prepared according to Example 1, mix them in a molar ratio of 1:1, and then dilute the total concentration of the synthetic peptide antigens to 50 μg / ml with sterile water for injection. The resulting antigen solution was filtered through a filter with a pore size of 0.2 μm and sterilized.
[0060] 1.2 Preparation of oil phase adjuvant
[0061] Sterilize the oil phase adjuvant at 50V at 121°C for 30min, and set aside.
[0062] 1.3 Emulsion of synthetic peptide vaccine
[0063] Clean the IKA emulsification equipment with 2000ml of sterilized distilled water for 3 times. Under the condition of 25-27°C, according to the volume ratio of the polypeptide antigen water phase and the adjuvant 1:1, first add the adjuvant into the emulsification ta...
Embodiment 3
[0065] Embodiment 3, Efficacy test of foot-and-mouth disease type O synthetic peptide vaccine
[0066] 1. Materials and methods
[0067] 1.1 Materials
[0068] 1.1.1 Synthetic peptide vaccine and control vaccine
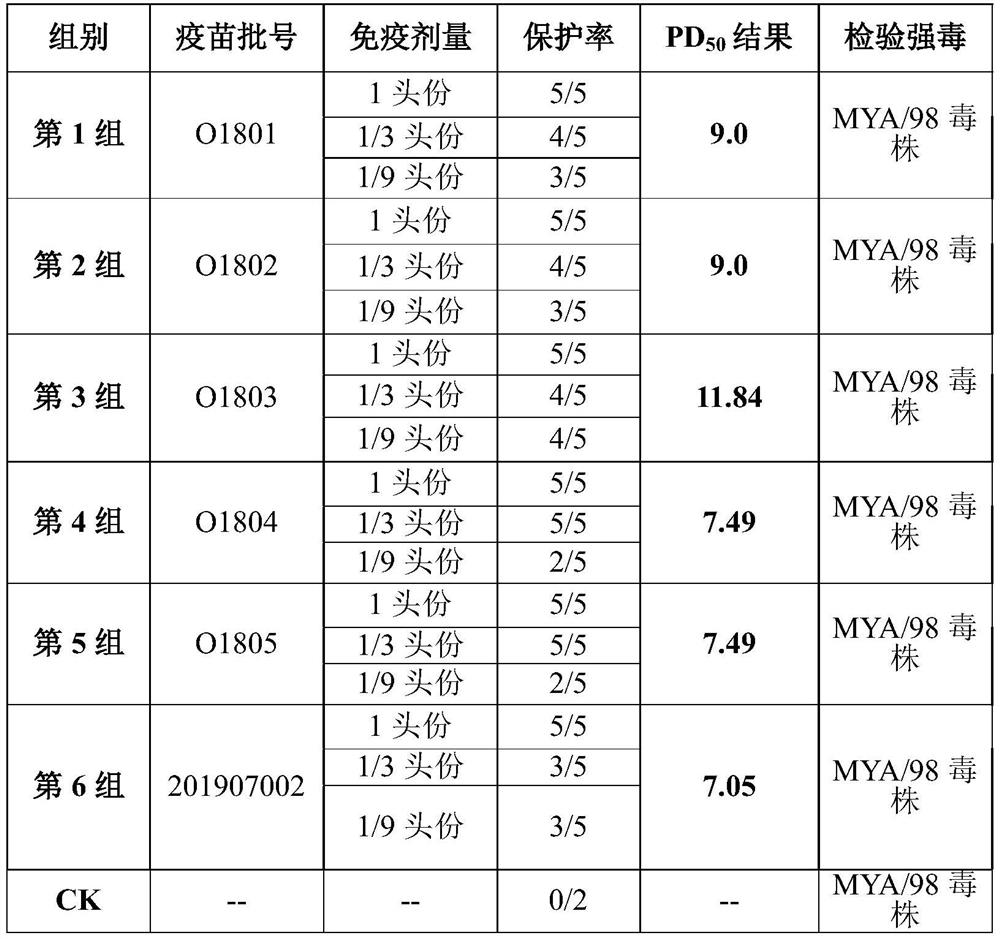
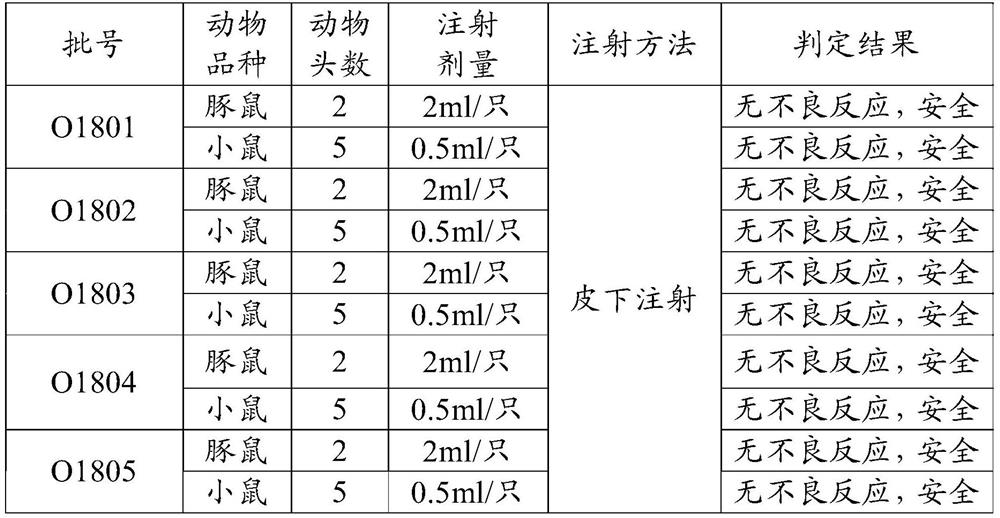
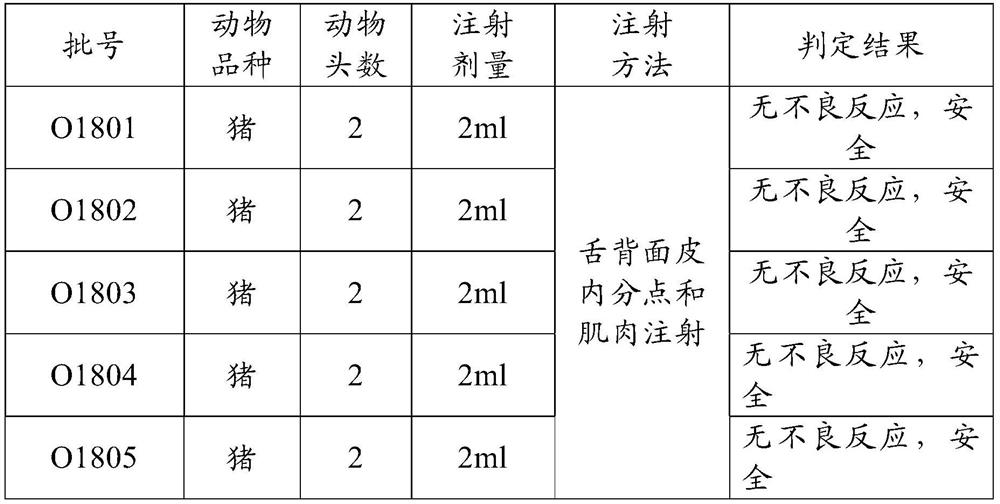
[0069] Prepare the polypeptide antigen such as polypeptide 1 (sequence 1) and polypeptide 2 (sequence 2) selected in embodiment 3 according to embodiment 1, then prepare three batches of foot-and-mouth disease containing polypeptide 1 and polypeptide 2 mixed components according to embodiment 2 Type synthetic peptide vaccines, the corresponding batch numbers are: O1801, O1802, O1803, and the corresponding batch number O1804 of the vaccine made of polypeptide 1 (sequence 1), and the corresponding batch number O1805 of the vaccine made of polypeptide 2 (sequence 2).
[0070] Control vaccine: porcine foot-and-mouth disease type O inactivated vaccine, batch number: 201907002
[0071] 1.1.2 Test animals
[0072] Select the same species, 4 months old, weighing about ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com