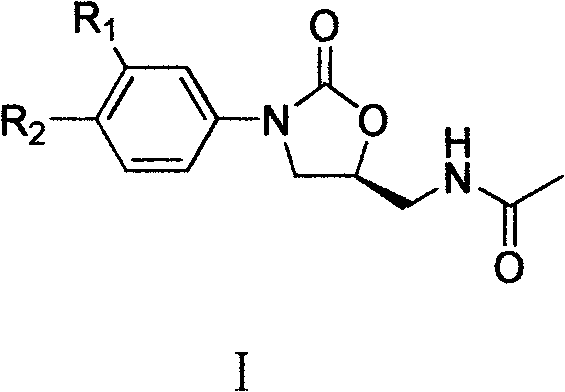
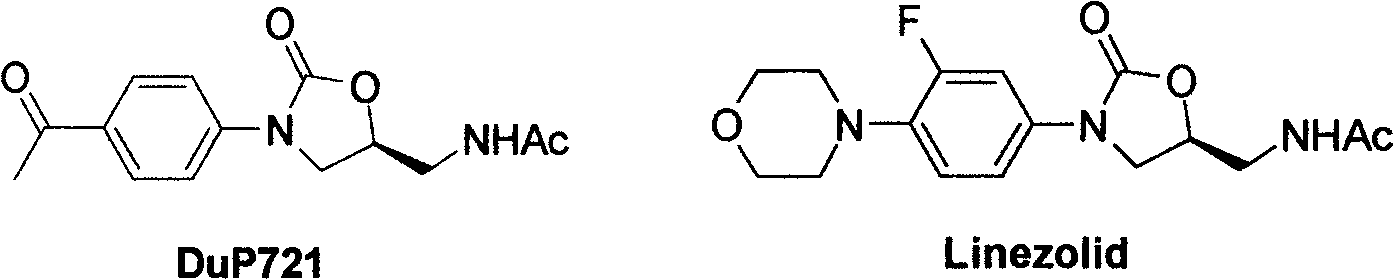
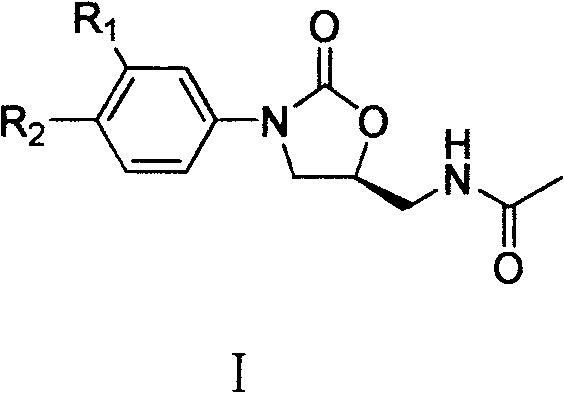
Cyanopyridyl-replaced oxazolidinone compound
A technology of oxazolidinones and compounds, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1 (S)-N-[[3-[3-fluoro-4-(2-amino-3-cyano-4-pyridyl)phenyl]-2-oxo-5-oxazolidinyl ]methyl]acetamide
[0084] Step 1 Preparation of (S)-1-amino-3-chloro-2-propanol hydrochloride
[0085] Dissolve 59.0g (0.56mol) of benzaldehyde in 150ml of ethanol, add dropwise 58.0ml (0.55mol) of ammonia water to the reaction solution, stir, and dropwise add 58ml (0.88mol) of (S)-epichlorohydrin to the reaction solution , Control the rate of addition to keep the reaction temperature below 40°C. After dropping, react at 35-40°C for 6h, then lower the reaction solution to room temperature and continue to react for 12h. Most of the solvent was distilled off under reduced pressure, and 100 ml of toluene was added. After stirring, the solution was raised to 35-40°C, and an aqueous hydrochloric acid solution (68ml hydrochloric acid, 77ml water) was added dropwise, and the solution was stirred at 35-40°C for 3h. Stand still, separate the water layer, wash the organic layer with water...
Embodiment 2
[0102] Example 2 (S)-N-[[3-[3-fluoro-4-(2-amino-3-cyano-6-pyridyl)phenyl]-2-oxo-5-oxazolidinyl ]methyl]acetamide
[0103] Step 1: (S,E)-N-[[3-[3-fluoro-4-[(3-dimethylamino-2-propenyl)yl]phenyl]-2-oxo-5-oxazolidine base] methyl] acetamide
[0104] S-N-[[3-(4-acetyl-3-fluorophenyl)-2-oxo-1,3-oxazolidin-5-yl]methyl]acetamide 5.0g (17mmol) and DMF- DMA13.6ml (102mmol) was added to 50ml of acetonitrile, heated to reflux and stirred for 7h. After the reaction was completed, the reaction solution was cooled to room temperature, suction filtered, and washed with acetonitrile to obtain 5.1 g of a yellow solid, with a yield of 86%, [M+H + ]: 350.1
[0105] Step 2: (S)-N-[[3-[3-fluoro-4-(2-amino-3-cyano-6-pyridyl)phenyl]-2-oxo-5-oxazolidinyl ]methyl]acetamide
[0106](S, E)-N-[[3-[3-fluoro-4-[(3-dimethylamino-2-propenyl)yl]phenyl]-2-oxo-5-oxazolidinyl] Add 5g (14.3mmol) of methyl]acetamide, 1.1g (17.2mmol) of malononitrile and 5.5g (71.6mmol) of ammonium acetate into 20ml of ethan...
Embodiment 3
[0108] Example 3 (S)-N-[[3-[3-fluoro-4-[2-(1-piperidinyl)-3-cyano-4-pyridyl]phenyl]-2-oxo- 5-Oxazolidinyl]methyl]acetamide
[0109] Step 1: (S)-N-[[3-[3-fluoro-4-(2-bromo-3-cyano-4-pyridyl)phenyl]-2-oxo-5-oxazolidinyl ]methyl]acetamide
[0110] Add 1.0 g (2.7 mmol) of the compound of Example 1 to 10 mL of pyridine, stir, cool to -10°C, and dropwise add 0.94 g (13.5 mmol) of sodium nitrite and 6.8 mL of concentrated sulfuric acid to the reaction solution in 6 mL of aqueous solution , Control the rate of addition so that the temperature of the reaction solution is -10°C. After dropping, the temperature of the reaction solution was raised to 0° C. for 1 h. After the reaction was completed, 1.94 g (16.2 mmol) of KBr in 3.5 mL aqueous solution was added to the reaction liquid, and the reaction was carried out at room temperature for 16 h. After the reaction is completed, filter with suction and wash the filter cake with water to obtain (S)-N-[[3-[3-fluoro-4-[2-(1-piperidyl)-3-c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com