Preparation method for trimethylethoxysilane
A technology of trimethylethoxysilane and trimethylchlorosilane, applied in the field of preparation of trimethylethoxysilane, can solve the problem of long process flow, low yield, difficulty in preparing trimethylchlorosilane, etc. problems, to achieve the effect of simplifying the process, high yield, reducing salt carry-out and volatilization loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
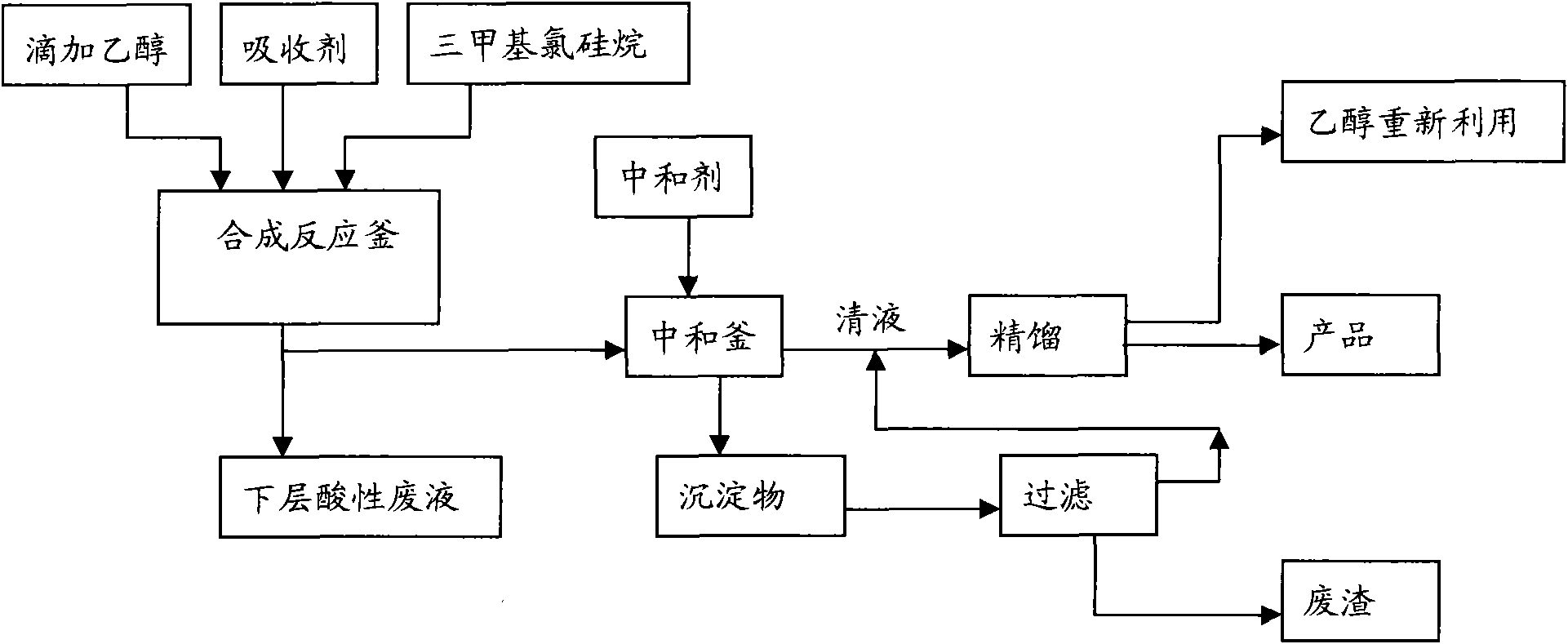
[0019] The present invention will be described in detail below in conjunction with the accompanying drawings. The description in this part is only exemplary and explanatory, and should not have any limiting effect on the protection scope of the present invention.
[0020] Adopt trimethylchlorosilane to prepare the reaction mechanism of trimethylethoxysilane as follows:
[0021] (1), the general formula of the alcoholysis reaction of organochlorosilane:
[0022] m en SiCl n-1 +(n-1)C 2 h 5 OH→M en Si(OC 2 h 5 ) n-1 +(n-1)HCl↑
[0023] where M en Is an alkane; n is 2, 3 or 4.
[0024] In this reaction, the more chlorine there is on the silicon, the easier it is for the first chlorine atom to react, the harder it is for the second chlorine atom, and so on. The fewer chlorine atoms on silicon, the more difficult it is to react. For example, the first chlorine of dimethyldichlorosilane can react smoothly at normal temperature and pressure, while the second chlorine need...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com