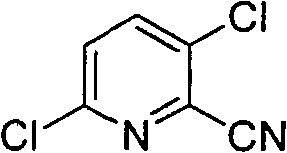
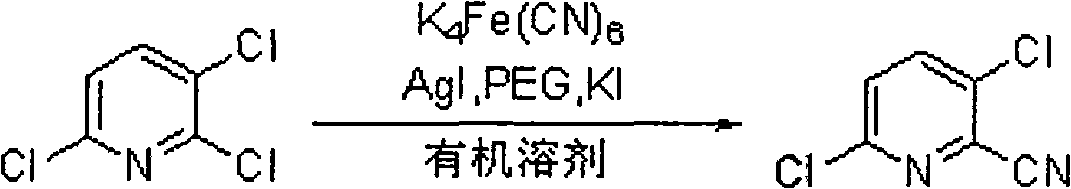Method for synthesizing 2-cyano-3,6-dichloropyridine
A dichloropyridine and synthesis method technology, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, organic chemistry, etc., can solve the problem of increased post-processing burden, low total yield, and environmental hazards and other problems, to achieve the effect of avoiding low total yield, reducing operating risks, and reducing the hazards of waste acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 2,3,6-trichloropyridine (9.1g, 50mmol), anhydrous potassium ferrocyanide (18.4g, 50mmol), silver iodide (1.7g, 5mmol), polyethylene glycol 200 (1.0g, 5mmol), Potassium iodide (0.8 g, 5 mmol) was dissolved in N,N-dimethylformamide (250 mL), and reacted under reflux at 200° C. for 12 hours with stirring. Ethyl acetate (50-80 mL) was added, the insoluble matter was removed by filtration, and the filtrate was washed twice with 20 mL of water. The organic phase was dried over anhydrous magnesium sulfate. The solvent was rotary evaporated to obtain 6.8 g of the product 2-cyano-3,6-dichloropyridine.
Embodiment 2
[0026] 2,3,6-trichloropyridine (9.1g, 50mmol), potassium ferrocyanide trihydrate (4.22g, 10mmol), silver iodide (0.2g, 1.5mmol), polyethylene glycol 400 (0.8g, 2mmol) , Potassium iodide (0.27g, 1.5mmol) was dissolved in toluene (150mL), and reacted under reflux at 120°C for 6 hours. Ethyl acetate (60-90 mL) was added. The insoluble matter was removed by filtration, and the filtrate was washed twice with 20 mL of water. The organic phase was dried over anhydrous magnesium sulfate. The solvent was rotary evaporated to obtain 6.1 g of the product 2-cyano-3,6-dichloropyridine.
Embodiment 3
[0028] 2,3,6-trichloropyridine (9.1g, 50mmol), anhydrous potassium ferrocyanide (1.84g, 5mmol), silver iodide (1.03g, 3mmol), polyethylene glycol 1000 (2.0g, 2mmol), Potassium iodide (0.27g, 1.5mmol) was dissolved in benzene (200mL), and reacted under reflux at 40°C for 2 hours with stirring. Ethyl acetate (70-100 mL) was added. The insoluble matter was removed by filtration, and the filtrate was washed twice with 20 mL of water. The organic phase was dried over anhydrous magnesium sulfate. The solvent was rotary evaporated to obtain 4.3 g of the product 2-cyano-3,6-dichloropyridine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com