EV71 virus neutralization epitope detection kit or reagent and preparation method thereof
A technology of EV71 and reagent kit, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve problems such as laborious, time-consuming, and unsatisfactory, and achieve the effects of wide applicability, guaranteed specificity, and reduced use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Preparation, purity and activity identification of EV71 purified solution
[0028] Inoculate Vero cells with C4 genotype EV71, harvest the culture fluid after culturing, purify EV71 virus after inactivation, and prepare EV71 purified fluid after sterilizing and filtering to obtain immune antigen. (See: Bishop NE, Hago DL, Borovec SV et al.Rapid and efficient purification of hepatitis Avirus from cell culture (rapid and efficient purification of hepatitis Avirus from cell culture). J VirolMethods, 1994, 47: 203-216)
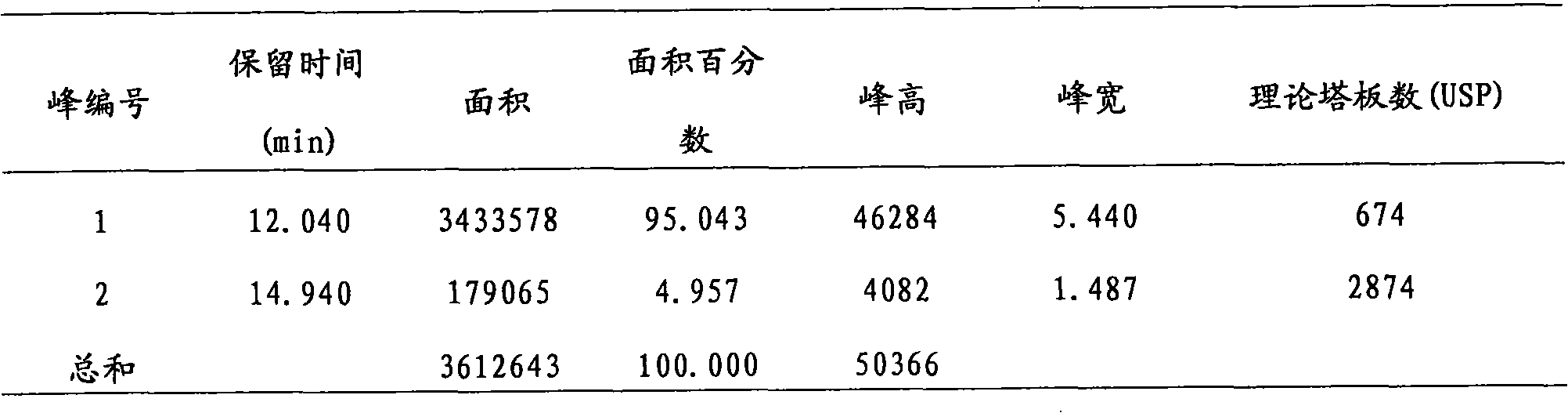
[0029] For the EV71 purified solution prepared above, the purity of its antigen was identified by HPLC method, and the result was greater than 95%, and the purified solution was placed at 2-8°C. Among them, the HPLC detection method includes the following process: use PBS to equilibrate the column for 40 minutes to the baseline level and then load the sample; use a sample needle to draw 100ul EV71 purified liquid, and inject it into the sample in...
Embodiment 2
[0033] Example 2: Preparation, titer detection and purification of anti-EV71 polyclonal antibody
[0034] The EV71 purified solution obtained in Example 1 was mixed and emulsified in equal volumes with complete Freund's adjuvant for immunization of New Zealand white rabbits, thereby preparing anti-EV71 serum (containing anti-EV71 polyclonal antibody).
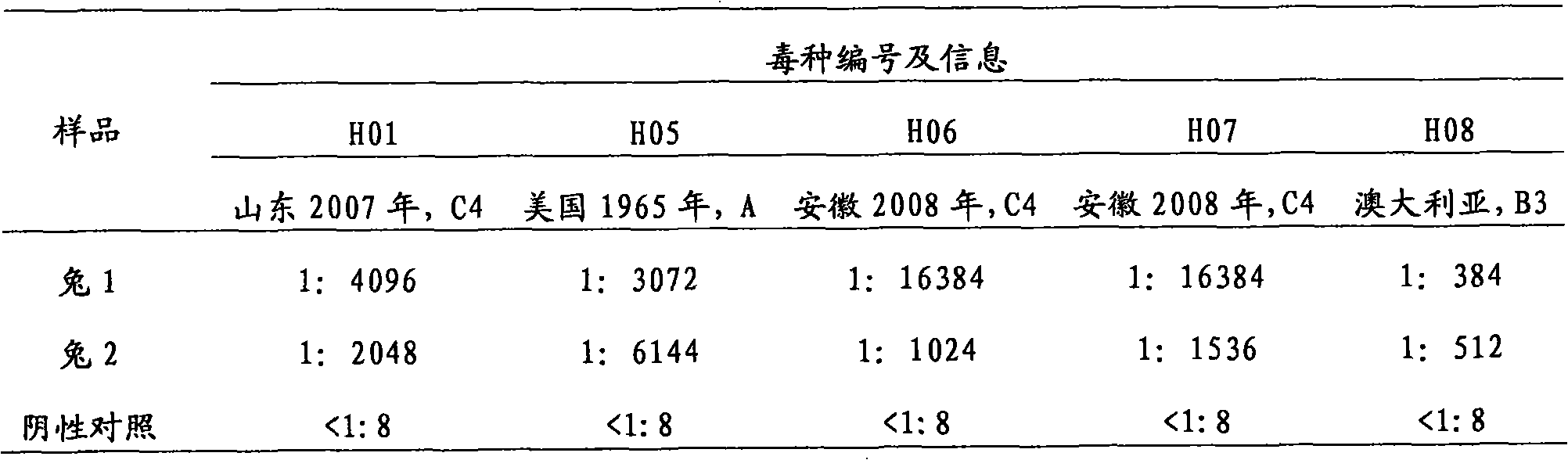
[0035] Use the indirect ELISA method to detect the antibody titer against the three genotypes A, B, and C of the anti-EV71 serum, and use the micro-neutralization assay to detect the neutralization test of the anti-EV71 polyclonal antibody against the three genotypes A, B, and C of the EV71 virus .
[0036] Taking the C4 genotype as an example, the indirect method titer detection of the anti-EV71 polyclonal antibody is carried out as follows: the EV71 purified solution obtained in Example 1 is diluted 100 times and coated on a microtiter plate (100ul / well), and coated with Overnight; wash the plate 3 times, block the microtite...
Embodiment 3
[0049] Embodiment 3: Preparation of anti-EV71 monoclonal antibody
[0050] BALB / c mice were immunized three times with EV71 purified solution, spleen cells from BALB / c mice with the highest titer by indirect ELISA were fused with SP2 / 0 myeloma cells in proportion, and fusion hybrid tumor cells were cultured on HAT medium . Subcloning was performed three times, and the indirect ELISA titer of each cell well was detected for each subcloning, and the wells with an OD value greater than 0.4 were cloned. Indirect detection method: Enzyme-labeled plates were coated with purified EV71 genotypes A, B, and C at a ratio of 1:100, cell culture supernatant was added, and goat anti-mouse IgG-HRP (Jackson Immunoresearch Company) was used as an enzyme-labeled reagent.
[0051] Finally, the inventor of the present application obtained an ideal hybridoma cell line through the above screening, and the applicant obtained the ideal hybridoma cell line on March 20, 2009 at the General Microorgani...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com