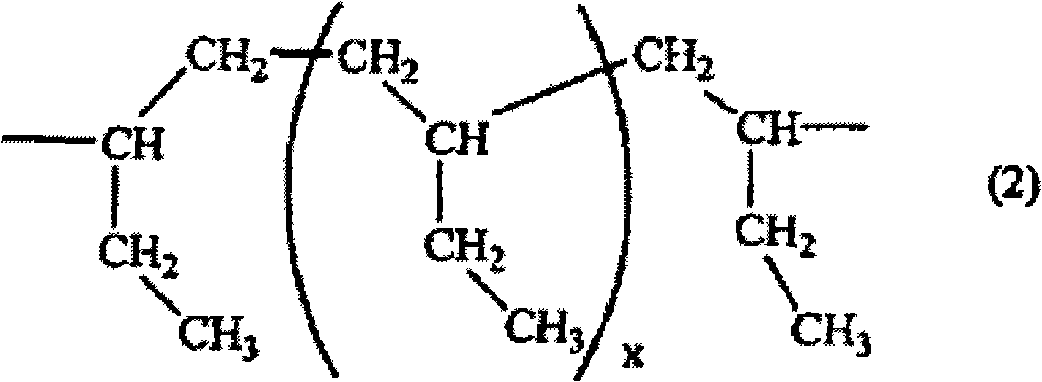
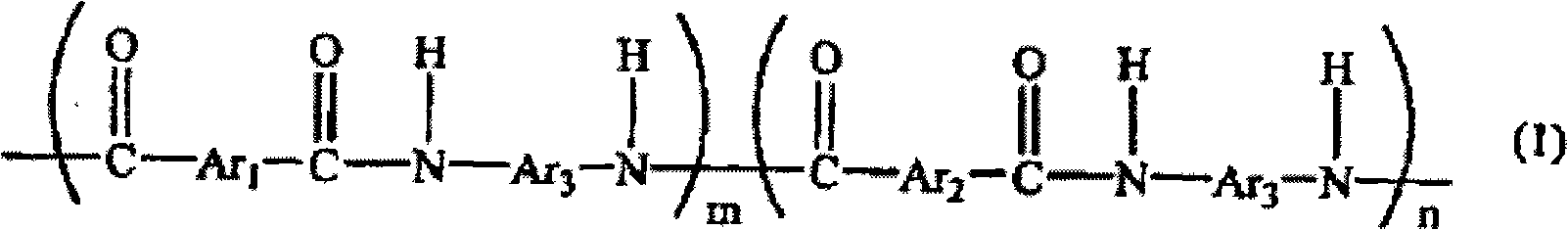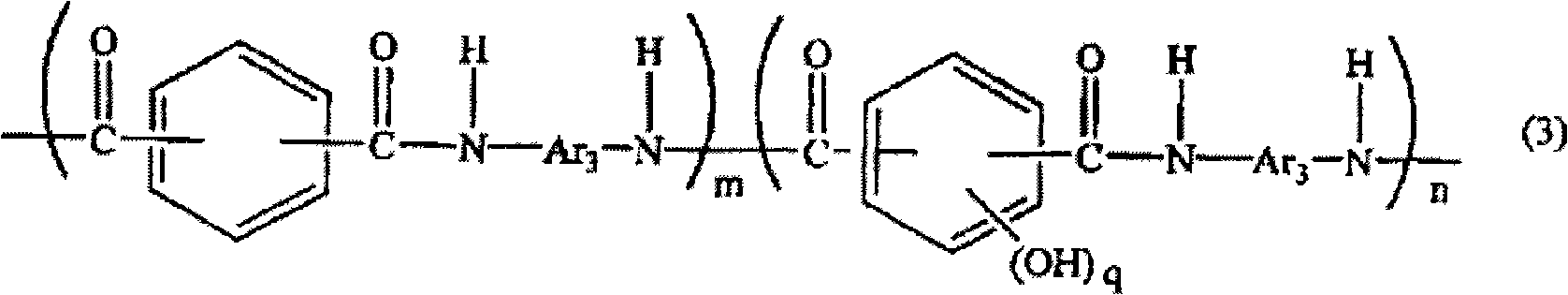Polyamide resin, epoxy resin composition using the same, and use of the composition
A polyamide resin, epoxy resin technology, applied in epoxy resin glue, synthetic resin layered products, applications, etc., can solve the problems of reduced film properties, insufficient flame retardancy or insufficient electrical properties, and achieve electrical reliability. Excellent, excellent flexibility and electrical reliability, excellent heat resistance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0070] Fill the flask with thermometer, condenser, stirrer with nitrogen, and add 1.8g (0.010mol) of 5-hydroxyisophthalic acid, 81.3g (0.490mol) of isophthalic acid, 102g (0.509mol) of 3,4'-diaminodiphenyl ether, 3.4 g of lithium chloride, 344 g of N-methyl-2-pyrrolidone, 115.7 g of pyridine. Next, after stirring and dissolving, 251 g (0.809 mol) of triphenyl phosphite was added and reacted at 90° C. for 8 hours to obtain a chain segment represented by the following formula (5) and having amino groups at both ends. The reaction solution of the polyamide resin (C-1). After cooling this reaction liquid to room temperature, it poured into 500 g of methanol, and the precipitated resin was filtered off, washed with 500 g of methanol, and purified by reflux of methanol. Next, after cooling to room temperature, it filtered and dried the filtrate to obtain a powdery resin (C-1). The obtained resin (C-1) was 160 g, and its yield was 96%. In addition, the molecular weight of the obta...
Embodiment 1
[0074] Fill the flask with thermometer, condenser, stirrer with nitrogen, and add 1.165g (0.006 mole) of 5-hydroxyisophthalic acid, 7.223g (0.043 mole) of isophthalic acid, 11.297g (0.056 mole) 3,4'-diaminodiphenyl ether, 0.955g of lithium chloride, 89.710g of N-methyl-2-pyrrolidone, and 35.678g of pyridine. Next, after stirring and dissolving, 28.330 g of triphenyl phosphite was added and reacted at 90° C. for 5 hours to obtain a polyamide resin having amine groups at both ends (the amine group equivalent weight is 1280 g / eq). Add 6.410 g of toluene and 6.410 g of N-methyl-2-pyrrolidone to this polyamide resin to dissolve 12.820 g (0.006 mol) of hydrogenated butadiene polymers having carboxyl groups at both ends (Nippon Soda Co., Ltd. CI-1000, the average molecular weight is 2142) and the resulting solution is further reacted for 3 hours to obtain an aromatic polyamide segment (a) comprising a segment represented by the following formula (6) and containing a phenolic hydroxyl...
Embodiment 2 and 3
[0078] Use the polyamide resin (C-1) that contains phenolic hydroxyl group that above-mentioned synthesis example 1 gains and the rubber modified polyamide resin (A-1) that contains phenolic hydroxyl group that embodiment 1 gains, formula (parts by weight) as shown in table 1 ) are mixed to obtain a varnish in which the epoxy resin composition of the present invention is dissolved in a solvent.
[0079] Table 1
[0080] Example 2
Example 3
A-1
44.54
28.37
C-1
18.80
NC-3000(*1)
4.45
2.84
GPH-65(*2)
1.01
2PHZ(*3)
0.22
0.14
NMP
200.0
200.0
[0081] *1: Manufactured by Nippon Kayakusha, a biphenyl-skeleton epoxy resin with an epoxy equivalent of 280 g / eq.
[0082] *2: Manufactured by Nippon Kayaku Co., a resin containing phenolic hydroxyl groups in the biphenyl skeleton, with an active hydrogen equivalent of 205 g / eq.
[0083] *3: Made by Shikoku Ch...
PUM
| Property | Measurement | Unit |
|---|---|---|
| epoxy equivalent | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com