Gemfibrozil polymorphism and preparation method thereof
A felozil crystal form and a technology for gemfibrozil, which is applied in the field of medicinal chemistry, can solve the problems of difficulty in storage, poor amorphous stability of gemfibrozil, etc., and achieve the effects of no reduction in drug content, good stability and easy production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 10 g of gemfibrozil to a mixed solvent composed of 5 mL of acetone and 2 mL of cyclohexane, heat (about 50 ° C) to reflux, stir until completely dissolved, transfer the solution to an ice-water bath (0 to 5 ° C) for crystallization, The solid was separated by filtration and dried in vacuum at 40° C. for 8 hours to obtain gemfibrozil in crystal form I.
Embodiment 2
[0021] Add 10g of gemfibrozil to 5mL of petroleum ether, heat (about 50°C) to reflux, stir until completely dissolved, crystallize in an ice-water bath at 0-5°C, separate by filtration to obtain a solid, and dry in vacuum at 40°C for 8 hours , to obtain I crystal form gemfibrozil.
Embodiment 3
[0023] Add 10g of gemfibrozil to a mixed solvent consisting of 2mL of ethanol and 10mL of cyclohexane, heat (about 50°C) to reflux, stir until completely dissolved, crystallize in an ice-water bath at 0-5°C, and separate by filtration to obtain a solid. °C and dried under vacuum for 8 hours to obtain crystal form I of gemfibrozil.
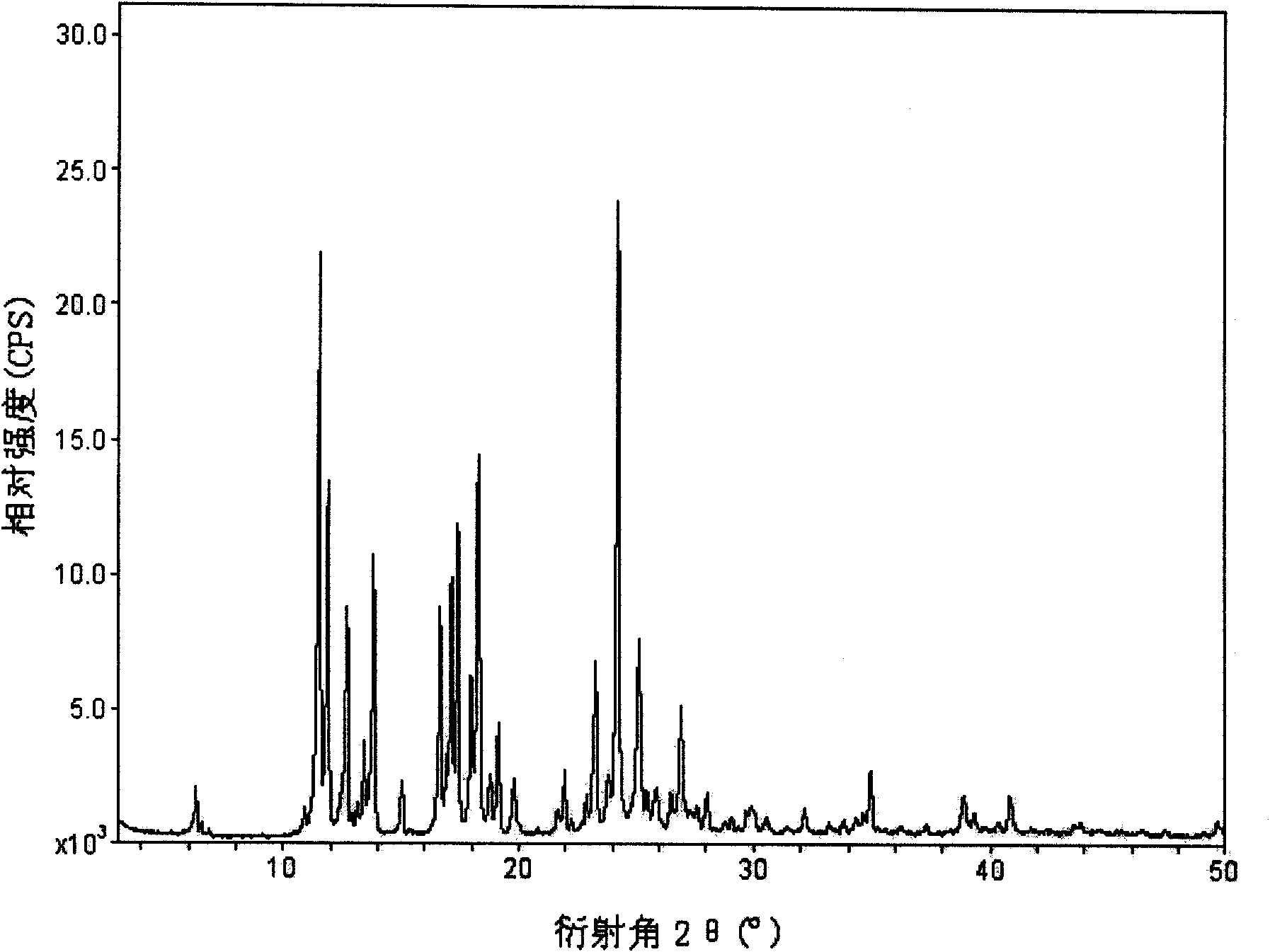
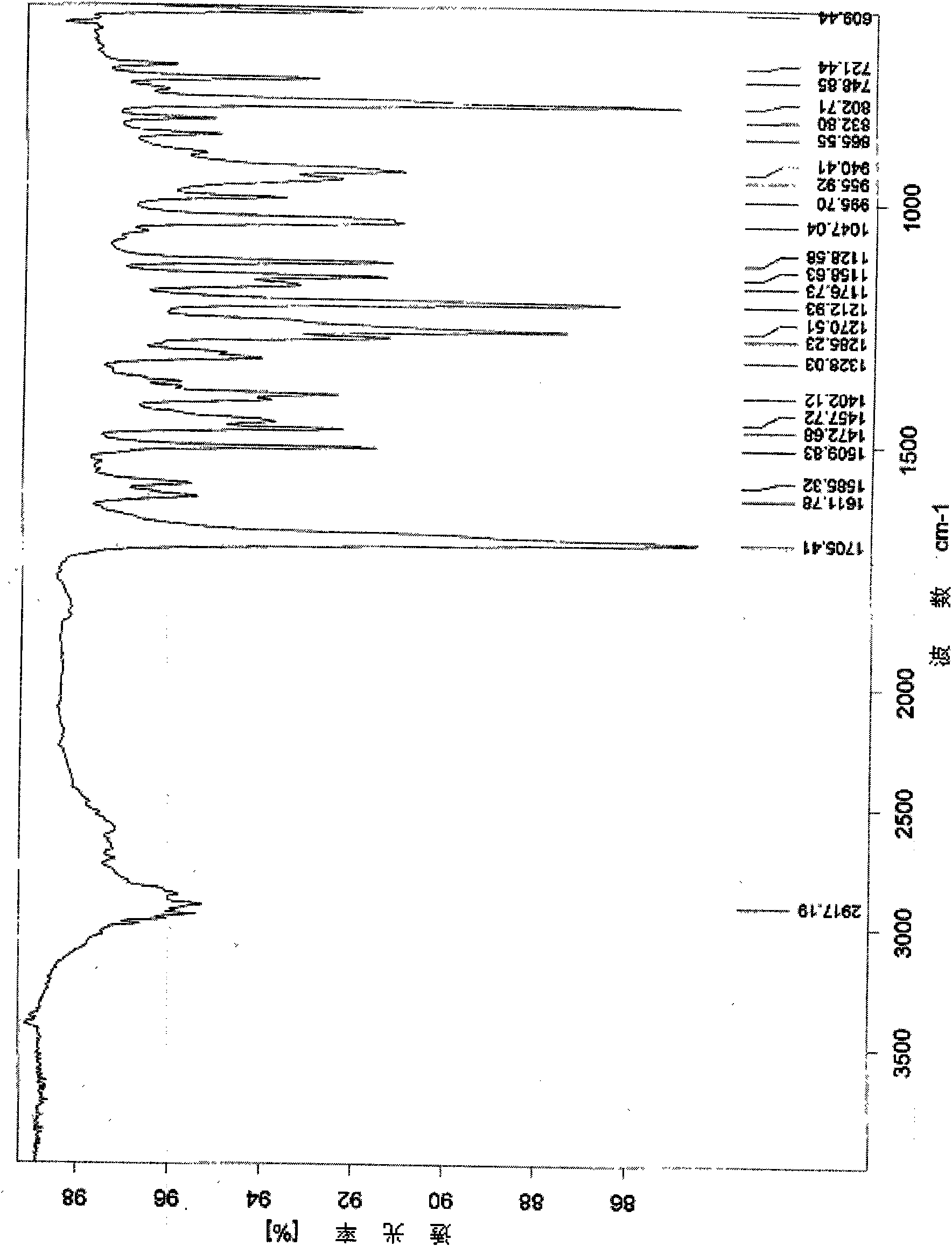
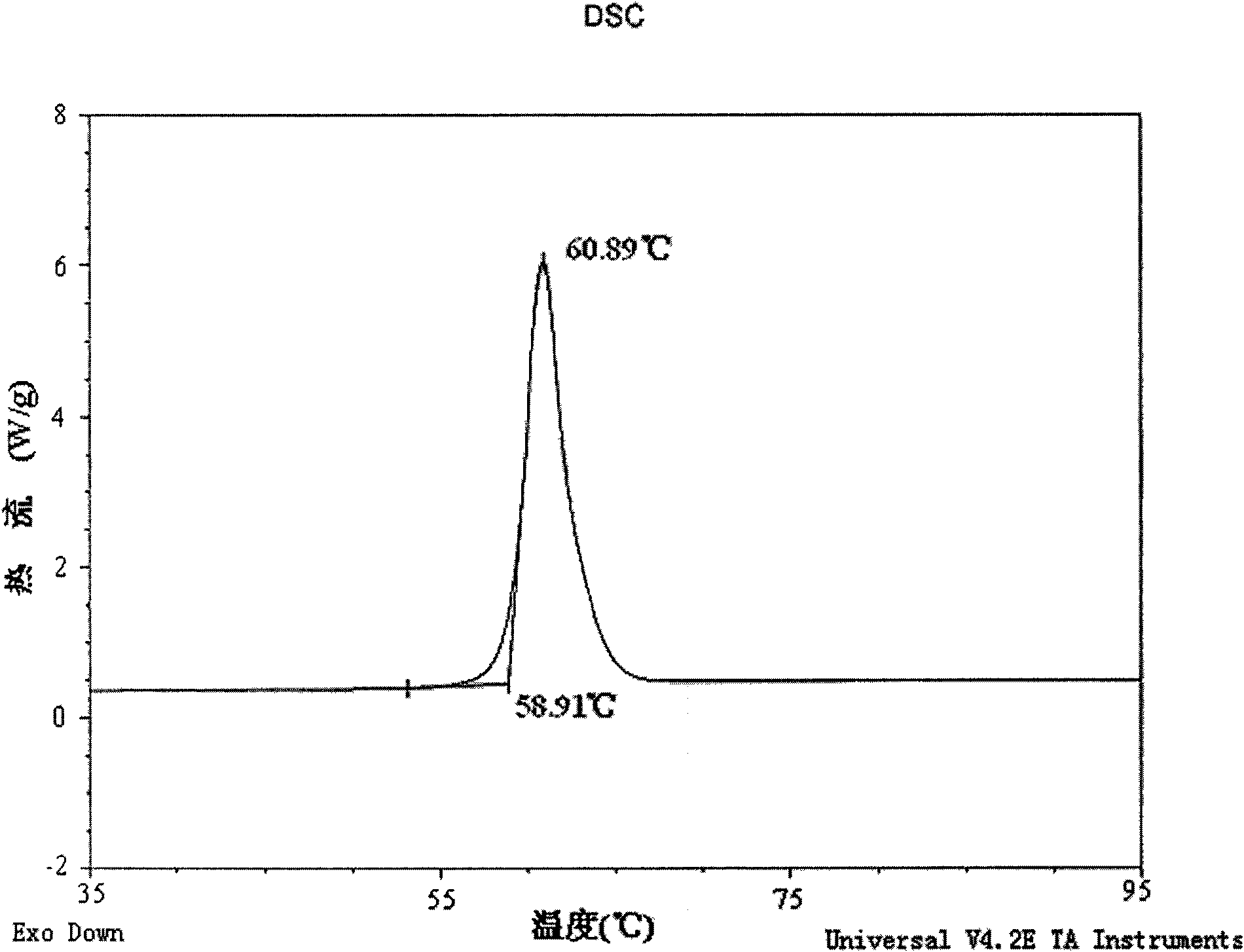
[0024] The X-ray powder diffraction pattern (XRD) of the gemfibrozil crystal form I of above-mentioned embodiment 1-3, see figure 1 , X-ray diffraction patterns of gemfibrozil crystals at 2θ (°, ±0.2) 6.32°, 11.52°, 11.89°, 12.69°, 13.45°, 13.83°, 15.02°, 16.65°, 17.14°, 17.39°, 17.99 °, 17.27°, 18.78, 19.13°, 19.79°, 21.95°, 23.27°, 24.23°, 25.12°, 26.92°, 34.94°, showing characteristic diffraction peaks. Infrared absorption spectrum wavenumber in potassium bromide (cm -1 ) for: 1705, 1509, 1472, 1402, 1285, 1270, 1212, 1158, 1128, 1047, 940, 802cm -1 wait, see attached figure 2 . There is an endothermic peak at 60.8°C in the differential th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com