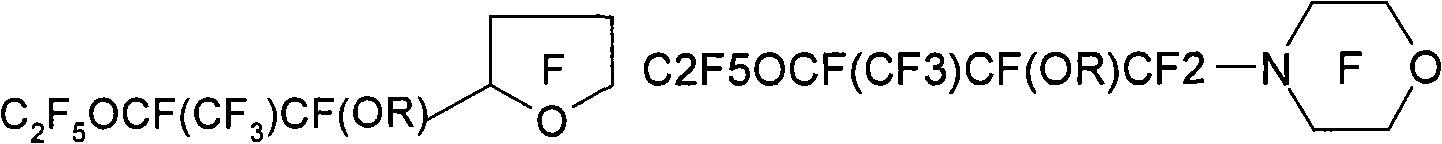Hydrofluoroether compounds and processes for their preparation and use
A technology of compound and hydrofluoroether, applied in the direction of ether preparation, chemical instruments and methods, lubricating composition, etc., can solve problems such as unsuitable and difficult alkylation reactions of perfluoroketones
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
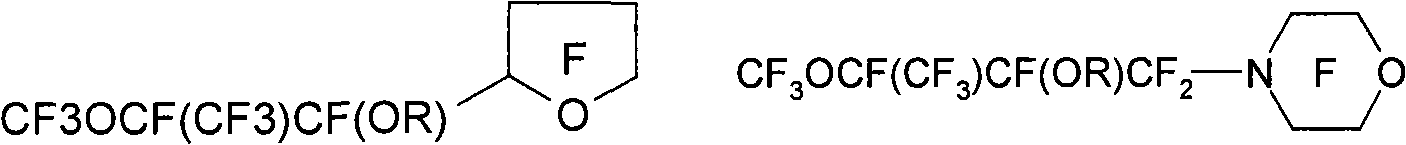
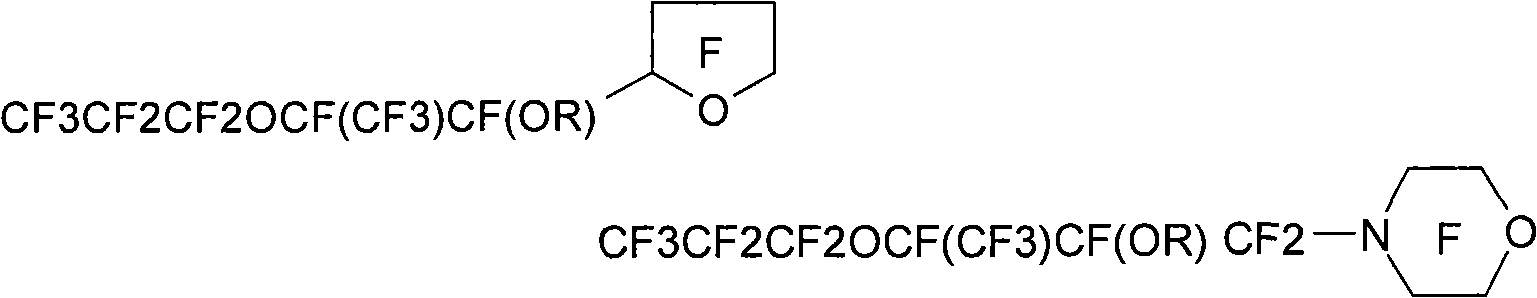
[0222] Preparation of hydrofluoroether compounds
[0223] The hydrofluoroether compound (HFE) of the present invention can be prepared by alkylation of a fluorine-containing alkoxide compound by subjecting a fluorine-containing ketone compound (more specifically, comprising independently A fluorine-containing ketone compound having two end groups of a fluoroalkyl or perfluoroalkyl group and an intervening oxytetrafluoroethylene moiety bonded to a carbonyl group through its central carbon atom, each Each of said terminal groups optionally contains at least one catenary heteroatom) with anhydrous alkali metal fluoride (for example, potassium fluoride or cesium fluoride) or anhydrous silver fluoride (preferably in anhydrous polar aprotic solvent Middle) prepared by reaction. See, for example, the preparation methods described in French Patent Publication No. 2,287,432 and German Patent Publication No. 1,294,949, as well as the methods detailed in US Patent No. 5,750,797 (Vitca...
example
[0340] Objects and advantages of this invention are further illustrated by the following examples, but the particular materials and amounts thereof recited in these examples, as well as other conditions and details, should not be construed to unduly limit this invention. These examples are illustrative only, and are not intended to limit the scope of the appended claims.
[0341] All parts, percentages, ratios, etc. in the examples and in the remainder of the specification are by weight unless otherwise indicated. Solvents and other reagents used were obtained from Sigma-Aldrich Chemical Company, St. Louis, MO (Sigma-Aldrich Chemical Company, St. Louis, MO) unless otherwise indicated.
[0342] In the following examples, a mixture of diastereomers is obtained due to the presence of two (or more) optical centers in the molecule. These diastereomers have boiling points very close to each other and therefore diastereomers cannot be separated by distillation. In some cases, howev...
example 1
[0373] preparation
[0374]
[0375] a- Preparation of intermediate ketones :
[0376]
[0377] Perfluoromorpholinoacetyl fluoride (94.6 g, 65% purity, 0.19 moles), C 3 f 7 OCF=CF 2 (50 g, 0.19 mol), anhydrous diglyme (75 g), potassium fluoride (2.2 g, 0.04 mol), stored in an oven at 125°C, and ground with a mortar and pestle just before use ) and phase transfer catalyst methyltributylammonium methylsulfate (1.2 g, 48% solution in diglyme) were mixed into a 600 mL Parr reaction vessel. The vessel was sealed, heated to 82°C, and maintained at this temperature for 16 hours. After cooling, the vessel was drained, the solid potassium fluoride was filtered, and the resulting biphasic reaction mixture was phase separated. The resulting ketone was purified to a purity of 92% by distillation (b.p. = 145-146°C). The IR spectrum shows 1790.4cm -1 CO absorption at the
[0378] b- Preparation of ethyl ether from intermediate ketones :
[0379] The intermediate ketone ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com