Sodium rabeprazole enteric-coated orally disintegrating tablets and preparation method thereof
A technology of rabeprazole sodium and orally disintegrating tablets, applied in the field of rabeprazole sodium enteric-coated orally disintegrating tablets and its preparation, can solve the problems of reduced bioavailability, drug inactivity, instability of proton pump inhibitors, etc. , to achieve the effect of improving utilization, good disintegration effect, and preventing children's dental caries
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Preparation method of rabeprazole sodium enteric-coated orally disintegrating tablets
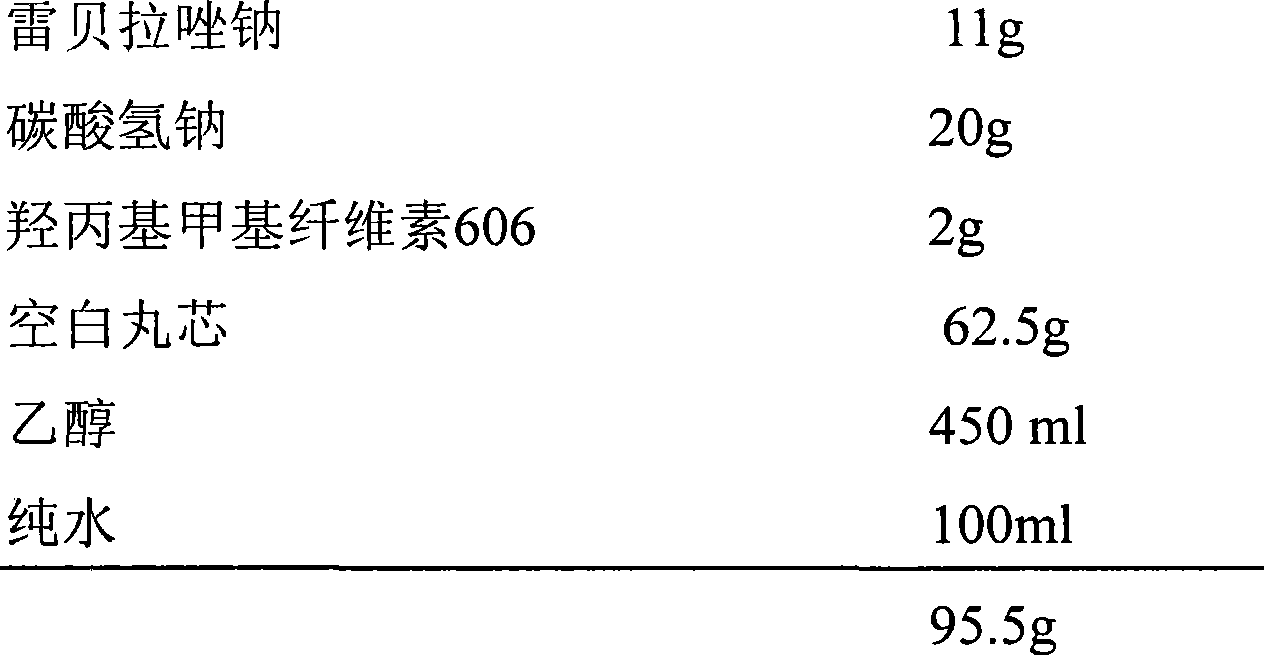
[0045] 1.1 Prescription:
[0046] First coating:
[0047]
[0048] Second coating:
[0049]
[0050]
[0051] The third coating:
[0052]
[0053] Tablets:
[0054]
[0055] 1.2 Preparation method:
[0056] (1) Coating for the first time: Dissolve rabeprazole sodium and sodium bicarbonate in 50ml of water, add 450ml of absolute ethanol, and set aside.
[0057] Place the blank pellet core in an inverted conical stainless steel fluidized bed with a bottom diameter of 188mm, a top diameter of 396mm, and a height of 1000mm. liquid atomizing gas. Set the frequency of the blower to 50HZ, the bed temperature of the fluidized bed to 40°C, the fluidization gas velocity to 2.0m / s, the flow velocity of the feed liquid to 0.5ml / min, and the atomizing pressure to 0.20mpa, start the fluidized bed, After the coating is complete, continue to keep warm for 10 minutes. ...
Embodiment 2
[0068] Example 2 Study on the release rate of rabeprazole sodium enteric-coated orally disintegrating tablets
[0069] 2.1 Instruments and reagents
[0070] 2.1.1 Instruments
[0071] ZRS-8G Intelligent Dissolution Tester (Tianjin Tianda Tianfa Technology Co., Ltd.); UV / VIS-4802S Double-beam UV-Vis Spectrophotometer (Shanghai Unic Instrument Factory), METLLE AE240 Electronic Balance (Mettlerto) Lido company), MP220 pH meter (Mettler Toledo).
[0072] 2.1.2 Reagent
[0073] Rabeprazole sodium reference substance (>99.5%) (batch number: 0805003, Xi’an Xintong Drug Research Co., Ltd.), rabeprazole sodium enteric-coated orally disintegrating tablets (specification: 10mg, batch number: 008025, Xi’an Xintong Drug Research Co., Ltd.), commercially available products: Rabeprazole Sodium Enteric-Coated Tablets (Polite) (Lot Number: 060873, Specification: 10mg, Misato Plant of Eisai Co., Ltd.), used in Reagents were all analytically pure.
[0074] 2.2 Methods and results
[0075] ...
Embodiment 3
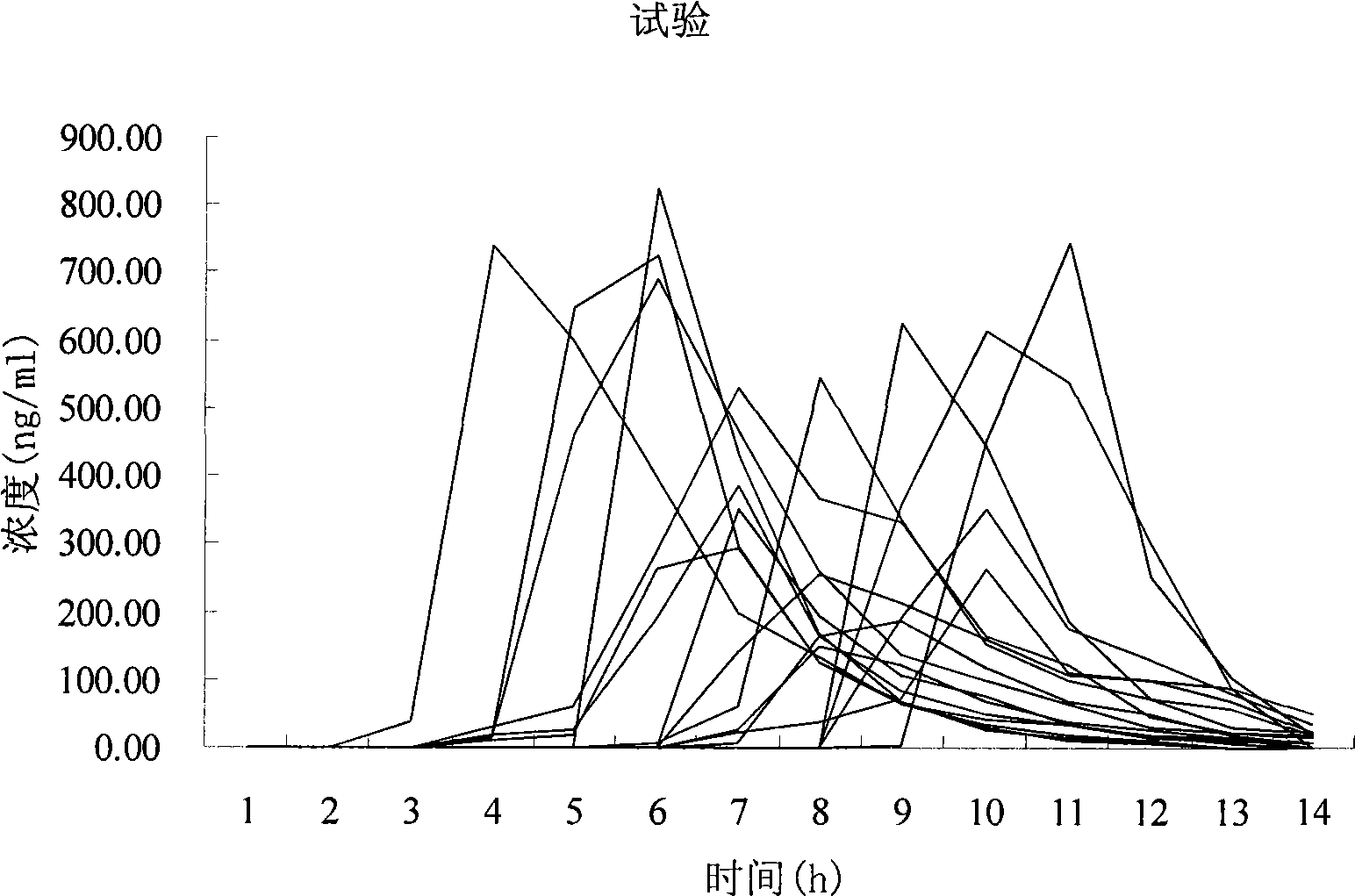
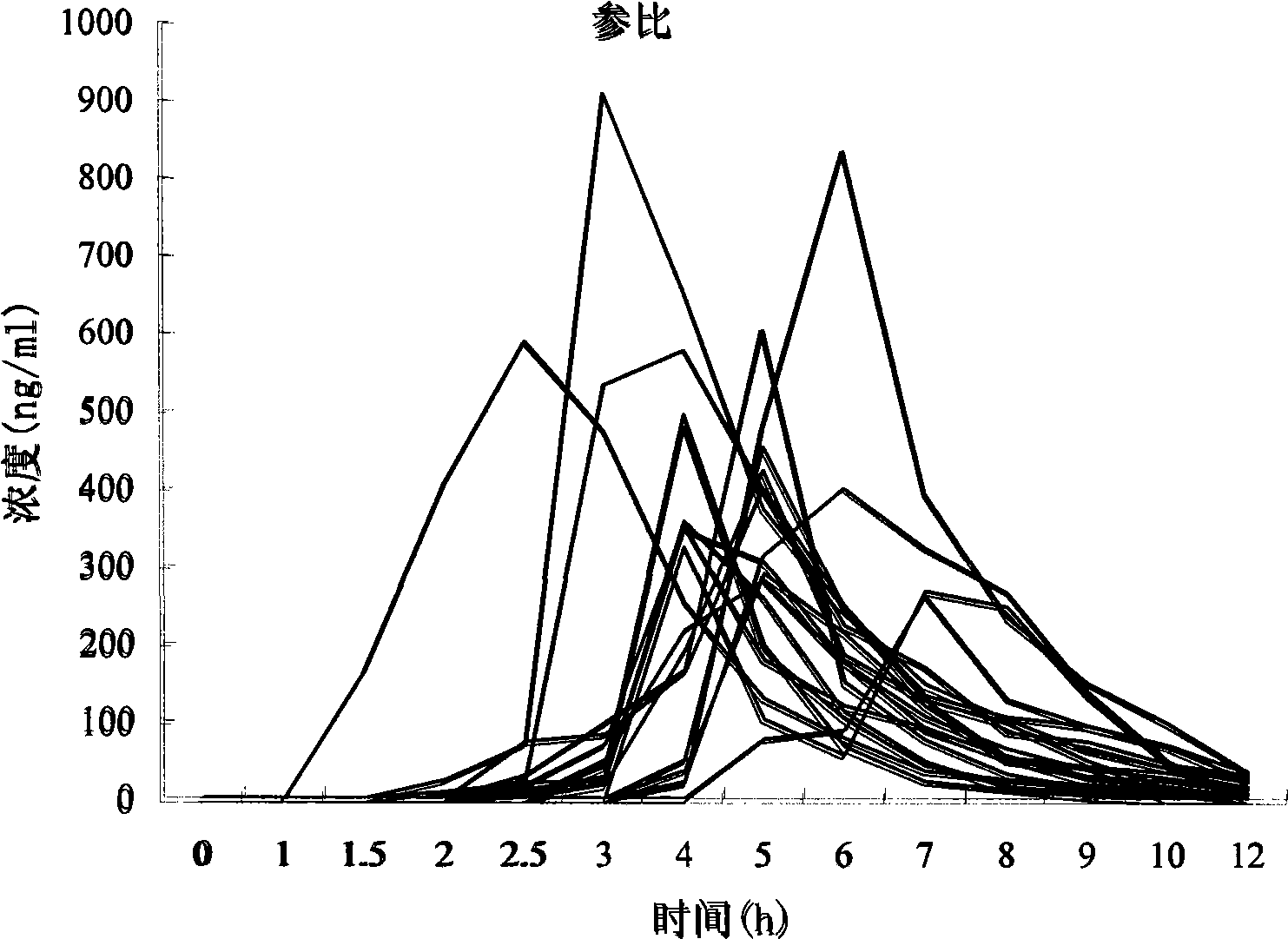
[0082] Example 3 Study on human bioavailability of rabeprazole sodium enteric-coated orally disintegrating tablets
[0083] 1.1 Research object:
[0084] 18 male volunteers, aged 21-30 years, height 166-178cm, weight within ±10% of the standard weight, no past medical history and drug allergy history, good mental state, liver, kidney function, electrocardiogram were normal.
[0085] After fasting for 10 hours, the subjects took the medicine on an empty stomach on the morning of the test day according to the prescribed dosage and method, and ate a unified standard meal 4 hours and 10 hours after taking the medicine. After taking the medicine, the subjects rested indoors. During the blood collection period, smoking, alcohol, caffeine and juice drinks were prohibited, and strenuous exercise was avoided.
[0086] 1.2 Drugs:
[0087] Test preparation: rabeprazole sodium enteric-coated orally disintegrating tablets (specification: 10mg, batch number: 008025, Xi'an Xintong Pharmaceut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com